UC Davis
UC Davis Previously Published Works
Title
1D lignin-based solid acid catalysts for cellulose hydrolysis to glucose and nanocellulose
Permalink
https://escholarship.org/uc/item/1t4608p8
Journal
ACS Sustainable Chemistry and Engineering, 3(10)
ISSN
2168-0485
Authors
Hu, S
Jiang, F
Hsieh, YL
Publication Date
2015-10-05
DOI
10.1021/acssuschemeng.5b00780
Peer reviewed
eScholarship.org Powered by the California Digital Library
University of California

1D Lignin-Based Solid Acid Catalysts for Cellulose Hydrolysis to
Glucose and Nanocellulose
Sixiao Hu, Feng Jiang, and You-Lo Hsieh*
Fiber and Polymer Science, University of California-Davis, One Shields Avenue, Davis, California 95616, United States
*
S
Supporting Information
ABSTRACT: One-dimensional (1D) solid acid catalysts have
been synthesized from lignin-based activated carbon fibers via
sulfonation and hydrothermal treatment to be mesoporous and
contain 0.56 mmol/g sulfonic and 0.88 mmol/g total acid for
direct hydrolysis of highly crystalline rice straw cellulose (CrI =
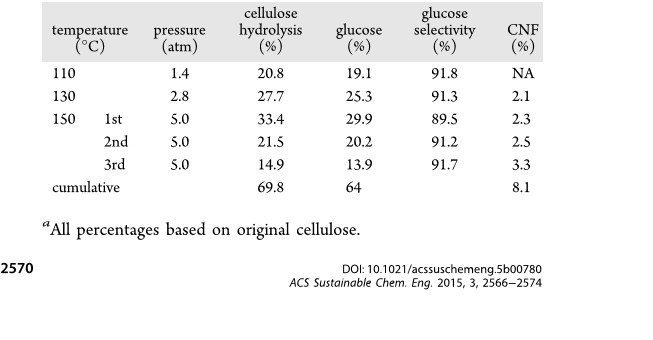
72.2%). Under optimal hydrothermal conditions of 150 °C
and 5 atm, 69.8% of cellulose was hydrolyzed in three
consecutive runs, yielding 64% glucose at 91.7 selectivity as
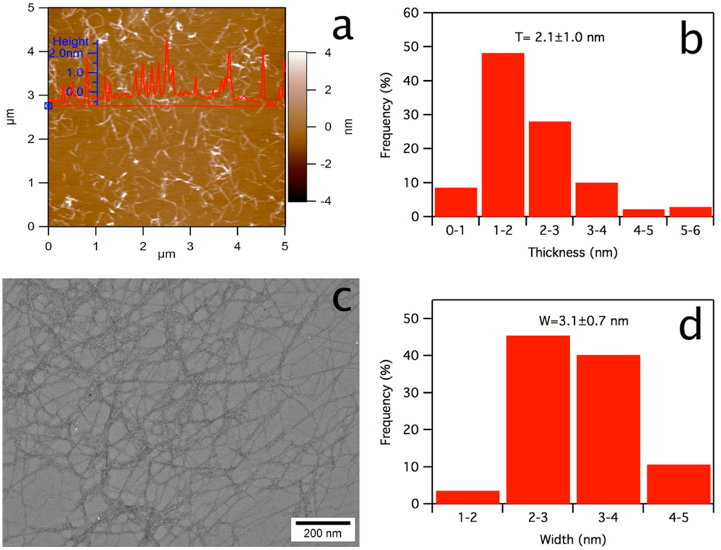
well as 8.1% cellulose nanofibrils (2.1 nm thick, 3.1 nm wide,
and up to 1 μm long). These 1D acid catalysts could be used
repetitively to hydrolyze the remaining cellulose as well as be
easily separated from products for hydrolysis of additional cellulose. In essence, complete valorization of rice straw cellulose has
been demonstrated by direct hydrolysis with these 1D acid catalysts to superior glucose selectivity while generating high value
cellulose nanofibrils.
KEYWORDS: Solid acid catalysts, Mesoporous, Activated carbon fibers, Hydrolysis, Glucose, Cellulose nanofibrils
■
INTRODUCTION
Heterogeneous solid acid catalysts are particularly suited for
large-scale industrial operations due to their many advantages
over homogeneous liquid phase catalysts. Heterogeneous solid
acid catalysts are noncorrosive and safe and can function more
efficiently under continuous flow than the typical batch
operations of homogeneous liquid acid catalysts.
1
In solid
acid catalysis, the reactants can be activated directly by protons
generated from the acid sites or via hydrogen spillover from
other active phases in the system.
2
The products could be easily
separated to allow recovery and reuse of the solid catalysts.
Various solid acid catalysts, such as zeolite,
3−6
silica,
7−12
hydrous zirconia,
12
nafion
13−15
and carbons,
16−19
have been
developed for common chemical reactions, including ester-
ification,
3,12,16
alcohol dehydration,
7,13
and hydrolysis.
18,19
Solid catalysts have also been reported in the hydrolysis of
cellulose for biofuel production, including nafion,
15
zeolite,
20
Fe
3
O
4
magnetic nanoparticles bearing mesoporous silica,
21
nano Zn-Ca-Fe oxide,
22
as well as a wide range of cellulose
chars and activated carbon particulates.
18,19,23−29
Among them,
carbon solid acid catalysts have exhibited particularly high
activity because of their high acid densities and strong
interactions with the β-1,4-glycosidic bonds in cellulose.
28
An
exceptional 74% glucose yield was achieved under hydro-
thermal conditions using sulfonated ordered mesoporous
carbon derived from a silica template.
18
However, either ball
milling
18,26,29
or ionic liquid dissolution of cellulose
27
was
necessary to enhance contact or diffusion to destruct the
crystalline structure of cellulose. To overcome the accessibility
issue, smaller Zn-Ca-Fe oxide nanoparticle catalysts have been
shown to significantly increase the hydrolysis of crystalline
cellulose into glucose and oligosaccharides.
22
Separation of
zero-dimensional nanocatalysts from the hydrolysate products
is, however, difficult. Therefore, solid acid catalysts with high
acid densities and yet geometries that allow easy separation for
reuse would be of significant interest. Furthermore, solid acid
hydrolysis of cellulose should generate nanocellulose, but this
has only been reported on solid cationic exchange polystyrene
resin beads (NKC-9 with 4.7 mmol/g acid density).
30
This study aimed to synthesize one-dimensional (1D) solid
acid catalysts for direct hydrolysis of crystalline cellulose for
simultaneous production of sugars and high valued nano-
cellulose. The 1D solid acid catalysts were prepared from
sulfonation of submicron-sized activated carbon fibers (ACFs)
using concentrated sulfuric acid. The ultrafine ACFs were
electrospun from lignin and simultaneously carbonized and
activated at 900 °C to high specific surface (1400 m
2
/g) and
pore volume (0.7 cm
3
/g).
31
The solid acid catalysts from lignin-
based ACFs have several advantages and desired attributes over
other solid acid catalysts for hydrolysis of crystalline cellulose.
Lignin, an abundant and underutilized biomass fraction, is an
excellent carbon precursor, and lignin chars are less recalcitrant
than rigid graphitic carbons, such as carbon nanotubes,
18
to be
readily sulfonated by concentrated sulfuric acid, a much safer
alternative than the commonly used fuming sulfuric acid for
sulfonation of graphitic carbons. These 1D solid acid catalysts
Received: July 29, 2015
Revised: August 21, 2015
Published: August 21, 2015
Research Article
pubs.acs.org/journal/ascecg
© 2015 American Chemical Society 2566 DOI: 10.1021/acssuschemeng.5b00780
ACS Sustainable Chem. Eng. 2015, 3, 2566− 2574

can be directly mixed with untreated semicrystalline cellulose.
ACFs, with the high aspect ratio, submicron diameters, and
porous structures are expected to be in more intimate contact
with cellulose, affording shorter access to the active sites and
better interconnected diffusive pathways for rapid and efficient
hydrolysis while minimizing deactivation of the catalysts. The
continuous fibrous form of the solid acid catalysts can be easily
separated from the soluble products via simple filtration for
repetitive use.
■
EXPERIMENTAL SECTION
Materials. Pure cellulose was isolated from rice straw (Calrose
variety) by extraction with 2:1 v/v toluene/ethanol and subsequent
dissolution of lignin and hemicellulose/silica with acidified NaClO
2
(1.4 wt %, 70 °C, 5 h) and alkaline KOH (5 wt %) at 90 °C for 2 h
resulting in 36% yield and 72.2% crystallinity.
32,33
Poly(ethylene
oxide) (PEO) (M
w
= 600 kDa), alkali lignin (low sulfonate) (AL
ls
)
(M
w
= 60 kDa, spruce origin) were acquired from Sigma-Aldrich
(USA), sodium hydroxide (anhydrous NaOH pallets, A.C.S. grade, 85
wt % minimum purity), aqueous NaOH (1N), sulfuric acid (95−98 wt
% purity), dinitrosalicylic acid (98 wt % purity), sodium sulfite
anhydrous (98 wt % minimum purity), potassium sodium tartrate
tetrahydrate (A.C.S. grade), and water (HPLC grade) were from
Fisher Scientific (USA). All of the chemicals were used as received.
Synthesis of Solid Acid Catalysts. Both carbon fibers (CFs) and
activated carbon fibers (ACFs) were synthesized according to a
previously reported approach.
31
Briefly, aqueous 9:1 w/w AL
ls
/PEO
(10 wt % total concentration) mixtures without and with NaOH at a
1:2 w/w NaOH/lignin ratio were electrospun into CF and ACF
precursor fibers, respectively. The precursor fibers were placed in a
quartz tube (2 cm inner diameter) of an electric furnace (Mini-Mite,
Lindberg/Blue), heated at 10 °C min
−1
under flowing N
2
at 100 mL
min
−1
to 105 °C, held for 0.5 h to drive off moisture, and then to 600
(CFs) or 900 °C (CFs and ACFs), held for another 0.5 h, and finally
cooled to ambient temperature under flowing N
2
at 100 mL min
−1
.
CFs and ACFs were then washed with deionized water repeatedly to
remove residual alkali metals and other small hydrocarbon impurities
followed by oven drying at 60 °C for 12 h.
Dried CFs and ACFs were sulfonated with concentrated sulfuric
acid at 110 or 150 °C for 20 h (5 mg/mL solid/liquid ratio, 1 atm) to
obtain sulfonated CF (SCFs) and ACFs (SACFs), respectively. SACFs
were rinsed thoroughly with 70 °C water to remove residual sulfuric
acid and then heated in water at 150 °C and 5 atm for 24 h using a 100
mL poly(tetrafluoroethylene) cylindrical reactor to obtain the
hydrothermal-treated SACFs. Hydrothermal-treated SACFs sulfonated
at 150 °C were further characterized and used in the hydrolysis of
cellulose and were denoted as HTSACFs unless specified otherwise.
Hydrolysis of Cellulose. Solid HTSACFs catalyst (160 mg) and
cellulose (40 mg) were dispersed in 32 mL of water in a 100 mL PTFE
cylindrical reactor, hermetically sealed, and heated in 110, 130, and
150 °C oil baths for 24 h with corresponding pressures of 1.4, 2.8, and
5.0 atm, respectively. The catalysts and remaining cellulose were
vacuum filtered (Whatman No. 1, dried, and weighed to 0.1 mg). The
average mass of the remaining cellulose and the catalysts (m
cell+HTSACF
)
from five reactions were used to determine the extent of cellulose
hydrolysis via eq 1. For repetitive hydrolysis, the dried HTSACF
catalysts along with the remaining cellulose were redispersed to 32 mL
of water and heated at 150 °C for a second and then a third time with
drying and weighing in between to determine the extent of cellulose
hydrolysis for each repetition. The filtrates were thoroughly dialyzed
(12−14 kDa MWCO, Fisher brand) to exchange the sugars in the
aqueous environm ent and retain nanocellulose in the dialysis
membrane. The glucose produced was quantified by the colorimetric
assay as described later. The nanocellulose yield was determined
gravimetrically by weighing dried nanocellulose and reported as a
percentage of the original cellulose.
=
−
×
+
m
cellulose hydrolysis (wt %)
160
40
100%
cell HTSACF
(1)
Analytical Methods. The chemical structures of ACFs, SACFs,
and HTSACFs were examined by Fourier transform infrared
spectroscopy (FTIR) (Nicolet 6700, Thermo Scientific), and their
morphologies were characterized by scanning electron microscope
(SEM) (FEI-XL 30, FEI) and atomic compositions by energy-
dispersive X-ray spectroscopy (EDX) adjunct to the SEM. All samples
were washed thoroughly with deionized water and dried at 60 °C for at
least 12 h before characterization. All FTIR spectra were collected
from samples pressed with anhydrous KBr powders into pellets. All
samples were sputter coated with gold for 1 min and imaged by SEM,
and noncoated samples were analyzed by EDX under a working
voltage of 5 kV. The acid density of the catalysts was analyzed via
conductometric titration using a pH/con ductivi ty meter (510,
OAKTON). For each titration, 10 mg of the acid catalysts were
dispersed in 15 mL of water, and 0.02 N NaOH was added to
neutralize the acids. The surface acid density (σ, in mmol/g of the
solid acid catalyst) was calculated from eq 2, where c is the NaOH
concentration (in N), m is the mass of the catalyst (in g), and V is the
volume of NaOH (in mL) used to neutralize the sulfonic acid and total
acids on the catalysts.
σ =
V
m
c
(2)
For surface area and pore characterization, the ACFs, SACFs, and
HTSACFs were dried at 50 °C for 48 h and then measured at 77 K by
a nitrogen adsorption−desorption analyzer (ASAP 2020, Micro-
metics). The single point total pore volume was estimated from
nitrogen adsorption at a relative pressure P/P
o
close to 1.The
Brunauer−Emmett−Teller (BET) surface area was calculated from the
isotherm in the BET linear region where relative pressure P/P
o
ranged
from 0.05 to 0.3. The mesopore surface area was derived from the
adsorption branch whereas pore and neck size distributions were
derived from both adsorption and desorption branches of the isotherm
using the Barret−Joyner−Halenda (BJH) method. To derive micro-
pore characteristics from the adsorption−desorption isotherm with
incomplete data below 0.05 P/P
o
relative pressure, micropore surface
area and pore hydraulic diameter distributions from 0.7 to 1.6 nm were
derived from a t-plot using the Mikhail, Brunauer, and Bodor MP
method
34
and the Harkins and Jura equation.
35
Micropore volume
(V
mp
) was derived from the tangent line of a contiguous range of the t-
plot using the surface area of the filled pores via eq 3
=
−+
×
+−
V
SS tt()()
2
15.47
nn nn
mp
11
(3)
where S
n
and t
n
are the surface area derived from the slope of the
tangent and the thickness of the adsorbed layer at the n point in the t-
plot, respectively, and 15.47 was the constant for converting gas
volume to liquid volume at STP.
The mixed HTSACF catalysts/cellulose (4:1) before and after three
consecutive reactions (32 mL of water and 150 °C) were sonicated in
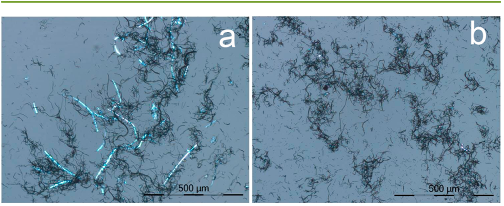
a water bath (Branson 2510) for 10 min and then visualized under a
polarizing light microscope (PLM, LEICA DM 2500). The nano-
cellulose separated from the catalyst by dialysis was diluted 10×,of
which 10 μL was deposited onto a freshly cleaved mica surface, air-
dried, and scanned using an atomic force microscope (AFM, MFP 3D,
Asylum Research) under ambient air conditions in the tapping mode
with OMCL-AC160TS standard silicon probes at 1 Hz scan rate and
512 × 512 pixel resolution. The height image and pro file were
processed with Igor Pro 6.21 loaded with MFP3D 090909 + 1409, and
the average thickness was determined from ∼100 individual
nanocellulose. Using a transmission electron microscope (TEM), a
diluted nanocellulose suspension (5 μ L) was deposited onto glow-
discharged carbon-coated grids (300 mesh copper, Formvar-carbon,
Ted Pella Incorporated, Redding, CA) with excess liquid being
removed by blotting with filter paper after 10 min, negatively stained
with 2 wt % uranyl acetate solution for 5 min, and observed using a
Philip CM12 TEM operated at 100 kV accelerating voltage. The width
ACS Sustainable Chemistry & Engineering Research Article
DOI: 10.1021/acssuschemeng.5b00780
ACS Sustainable Chem. Eng. 2015, 3, 2566− 2574
2567

of the nanocellulose was measured from ∼100 individual nanofibrils
using an image analyzer (ImageJ, NIH, USA).
The water-soluble hydrolysates (10 μL) were injected into a 0.32
mm × 100 mm Hypercarb column, and the compositions were
analyzed by LC-MS (Thermo Scientific, USA). A standard reverse-
phase gradient utilizing 5 mM ammonium formate as solvent “A” and
acetonitrile as solvent “B” was run at a 25 μL/min flow rate for 12 min.
The eluent was monitored for negative ions by an orbitrap XL
(Thermo Scientific, USA) operated in the centroid mode at 4.5 kV
spray voltage, 275 °C capillary temperature, and 20 sheath gas setting.
Spectral data were acquired at a 30,000 fwhm resolution setting with
the lockmass feature, which has a typical mass accuracy of <2 ppm.
The glucose concentration was derived from the colorimetric assay.
36
Briefly, 100 mL of 1 wt % dinitrosalicylic acid reagent (DNS) solution
was prepared by mixing 1 g of dinitrosalicylic acid, 1 g of sodium
hydroxide, and 50 mg of sodium sulfite. Then, 1.5 mL of DNS solution
and 1.5 mL of hydrolysate were mixed and then heated at 90 °C for 10
min to develop the red-orange color, which was stabilized by
subsequently adding 1 mL of 40 wt % potassium sodium tartrate
solution. U pon cooling to ambient temperature, the UV− vis
absorbance of the stabilized solution was measured (Evolution 600,
Thermo Scientific) at 575 nm. Their sugar concentrations were
derived from the standard calibration using glucose solutions in 0.125
to 1.25 mg/mL concentrations (Figure S1). The glucose yield and
selectivity were calculated based on the original cellulose (40 mg) via
eqs 4 and 5, respectively, where C
g
is the glucose concentration (mg/
mL) determined by colorimetric titration and V
g
is the volume of the
hydrolysate (mL).
=
×
×
CV
glucose yield (%)
40
100%
gg
(4)
=×glucose selectivity (%)
glucose yield
cellulose hydrolysis
100%
(5)
■
RESULTS AND DISCUSSION
Acid Density and Chemical Structures of Solid Acid
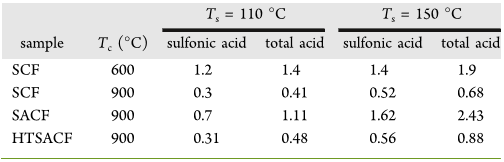
Catalysts. Sulfonated carbon fibers (SCFs) and sulfonated
activated carbon fibers (SACFs) were prepared under two
carbonization (T
c
= 600 or 900 °C) and sulfonation (T
s
= 110
or 150 °C) temperatures (Table 1). The SACFs carbonized at
900 °C were also hydrothermal-treated (150 °C, 5 atm, 24 h)
to obtain HTSACFs. The acid densities were determined by
conductometric titration where conductivity typically decreased
sharply from neutralization of sulfonic acid groups, then stayed
constant as the weaker carboxylic acid was consumed, and
finally increased after all acid sites were neutralized with NaOH
addition (Figure S2). In all titration curves, sulfonic acid was
shown to be dominant with much lower extents of carboxylic
acid groups. Although sulfonic acid is expected from the
sulfonation reaction, carboxylic acid groups are likely from
oxidation of the reductive hydroxyl groups during prolonged
sulfonation.
Both sulfonic and total acid densities were higher for SCFs
carbonized at the lower 600 °C T
c
and for all fibers sulfonated
at the higher 150 °C T
s
(Table 1). SCFs carbonized at the
higher 900 °C had approximately 1/4 to 1/3 of the respective
sulfonic and total acid densities, i.e., 0.3 and 0.41 mmol/g at T
s
= 110 °C and 0.52 and 0.68 mmol/g at T
s
= 150 °C,
respectively, of those carbonized at 600 °C. Sulfonation of
ACFs (T
c
= 900 °C) at the respective T
s
of 110 and 150 °Con
SACFs, on the other hand, doubled and tripled the sulfonic and
total acid quantities relative to the corresponding SCFs.
Although the more rigid carbon structures produced at the
higher carbonization temperature of 900 °C are more difficult
to be sulfonated, hence lower acid densities, higher specific
surface ACF could only be produced at 900 °C. The greater
sulfonation effects on ACFs are due to their higher internal
surfaces as well as the carbon microstructure defects introduced
by alkali hydroxide activation.
37
Most significantly, the higher
sulfonation temperature produced increases in sulfonic and
total acids on the activated SACFs that were more pronounced
than on the SCFs. Overall, the SACFs carbonized at higher 900
°C still had a much higher acid density than SCFs carbonized at
lower 600 °C and therefore were hydrothermally treated to
prepare stable solid acid catalysts. Following hydrothermal
washing (150 °C, 5 atm, 24 h), the sulfonic and total acid
densities of HTSACFs sulfonated at 110 and 150 °C declined
significantly by 55% (0.31 and 0.48 mmol/g) and 65% (0.56
mmol/g and 0.88 mmol/g), whereas their mass decreased to
lesser degrees of 5 and 9%, respectively. The substantial losses
of sulfonic and total acids coupled with some mass losses from
hydrothermal washing suggest that sulfonation of the pore
surfaces may have led to fragmentation and solubilization of
small hydrocarbons. Both surface acid densities and catalyst
mass remained unchanged following a second hydrothermal
washing, showing no further leaching. That these acid sites
remain stable under the hydrothermal catalysis condition is a
critical attribute for HTSACF solid acid catalysts to be effective
in repetitive use. HTSACFs sulfonated at 150 °C with higher
sulfonic (0.56 mmol/g) and acid (0.88 mmol/g) densities was
used as the solid acid catalyst for the hydrolysis of cellulose to
be described later.
The presence of carboxylic and sulfonic acid groups on
ACFs, SACFs, and HTSACFs was further confirmed by FTIR
and EDX. Both FTIR spectra of SACFs and HTSACFs showed
aCO peak at 1720 cm
−1
but no evidence of the C−OH peak
at 1630 cm
−1
(Figure 1a), indicating complete oxidation of C−
OH to carbonyl and carboxylic acid by the concentrated
sulfuric acid. Aromatic skeletal st retching at 1580 cm
−1
,
expected from the polycyclic aromatic structures in all three
activated carbons, was also clearly present. In addition, EDX
showed the O content to be tripled from 4.4 wt % in ACFs to
12.7 wt % in SACFs in which 2.8 wt % of S was also detected
(Figure 1b). The hydrothermal treatment significantly lowered
the respective O and S contents in SACFs to 7.3 and 0.9 wt %
in HTSACFs. Such elemental com position changes were
consistent with the decreases in sulfonic and total acid densities
presented previously and attributed to the dissolution of the
highly oxidized and sulfonated hydrocarbons from the
hydrothermal treatment.
Morphologies and Porous Structures of the Solid
Acid Catalysts. ACFs were 500 nm to 2 μm wide and most
were over 100 μm long (Figure 2a and b). Sulfonation caused
SACFs to fragment into a few tens of micrometers in length.
This obvious length reduction is attributed to the extreme
Table 1. Sulfonic and Total Acid Density (mmol/g) of
Sulfonated Carbon Fibers (SCFs) and Sulfonated Activated
Carbon Fibers (SACFs) Prepared at the Prescribed
Carbonization (T
c
) and Sulfonation (T
s
) Temperatures
T
s
= 110 °C T
s
= 150 °C
sample T
c
(°C) sulfonic acid total acid sulfonic acid total acid
SCF 600 1.2 1.4 1.4 1.9
SCF 900 0.3 0.41 0.52 0.68
SACF 900 0.7 1.11 1.62 2.43
HTSACF 900 0.31 0.48 0.56 0.88
ACS Sustainable Chemistry & Engineering Research Article
DOI: 10.1021/acssuschemeng.5b00780
ACS Sustainable Chem. Eng. 2015, 3, 2566− 2574
2568

oxidation in concentrated sulfuric acid and the mechanical
forces from ma gnetic agitation (Figure 2c and d). The
hydrothermally washed HTSACFs remained similarly frag-
mented but showed no further morphological changes (Figure
2e and f). All three fibers, i.e., ACFs, SACFs, and HTSACFs,
appeared similarly porous with surface macropores in the tens
of nanometers range (Figure 2b, d, and f).
The porous structures of ACFs, SACFs, and HTSACFs were
further analyzed by BET nitrogen adsorption to all exhibit type
IV adsorption − desorption isotherms (Figure 3a) typical of
microporous and mesoporous materials. Each showed a step-
down at 0.45 relative pressure in the desorption isotherm
(Figure 3a) and an artificial peak at 4 nm (Figure 3b),
suggesting an ink-bottle porous structure with a neck size
smaller than 5 nm.
38,39
The sulfonation reaction slightly and
hydrothermal treatment more prominently shifted the meso-
pores to larger pore sizes and wider ranges ( Figure 3b and c).
Sulfonation slightly increased the mesopore (5−30 nm) surface
area and volume from 407 m
2
/g to 416 m
2
/g and from 0.71 to
0.75 cm
3
/g, respectively (Figure 3c), but significantly decreased
micropore (0.5−0.8 nm) surface area and volume from 444 to
305 m
2
/g and 0.18 to 0.13 cm
3
/g, respectively (Figure 3d),
resulting in a slight decrease in the total BET surface area (885
to 752 m
2
/g) and pore volume (0.9 to 0.88 cm
3
/g).
Figure 1. (a) FTIR and (b) EDX of ACF, SACF, and HTSACF. Inset in (b): elemental composition (wt %).
Figure 2. SEM of (a,b) ACFs, (c,d) SACFs, and (e,f) HTSACFs.
ACS Sustainable Chemistry & Engineering Research Article
DOI: 10.1021/acssuschemeng.5b00780
ACS Sustainable Chem. Eng. 2015, 3, 2566− 2574
2569