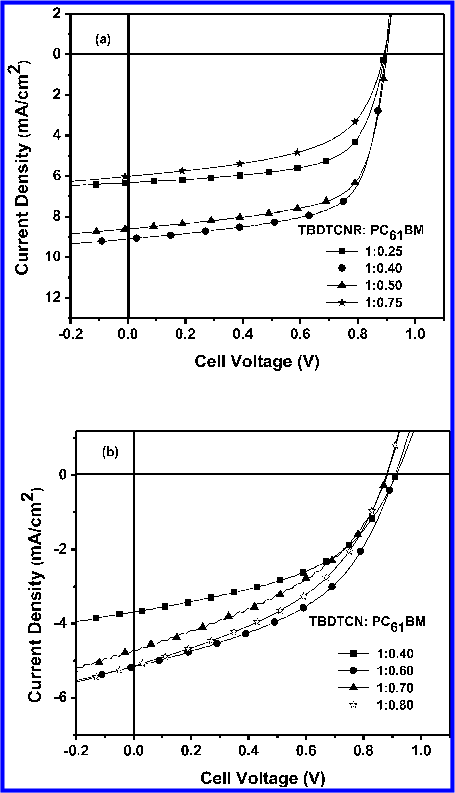
Abstract: There has been an intensive search for cost-effective photovoltaics since the development of the first solar cells in the 1950s. [1–3] Among all alternative technologies to silicon-based pn-junction solar cells, organic solar cells could lead the most significant cost reduction. [4] The field of organic photovoltaics (OPVs) comprises organic/inorganic nanostructures like dyesensitized solar cells, multilayers of small organic molecules, and phase-separated mixtures of organic materials (the bulkheterojunction solar cell). A review of several OPV technologies has been presented recently. [5] Light absorption in organic solar cells leads to the generation of excited, bound electron– hole pairs (often called excitons). To achieve substantial energy-conversion efficiencies, these excited electron–hole pairs need to be dissociated into free charge carriers with a high yield. Excitons can be dissociated at interfaces of materials with different electron affinities or by electric fields, or the dissociation can be trap or impurity assisted. Blending conjugated polymers with high-electron-affinity molecules like C60 (as in the bulk-heterojunction solar cell) has proven to be an efficient way for rapid exciton dissociation. Conjugated polymer–C60 interpenetrating networks exhibit ultrafast charge transfer (∼40 fs). [6,7] As there is no competing decay process of the optically excited electron–hole pair located on the polymer in this time regime, an optimized mixture with C60 converts absorbed photons to electrons with an efficiency close to 100%. [8] The associated bicontinuous interpenetrating network enables efficient collection of the separated charges at the electrodes. The bulk-heterojunction solar cell has attracted a lot of attention because of its potential to be a true low-cost photovoltaic technology. A simple coating or printing process would enable roll-to-roll manufacturing of flexible, low-weight PV modules, which should permit cost-efficient production and the development of products for new markets, e.g., in the field of portable electronics. One major obstacle for the commercialization of bulk-heterojunction solar cells is the relatively small device efficiencies that have been demonstrated up to now. [5] The best energy-conversion efficiencies published for small-area devices approach 5%. [9–11] A detailed analysis of state-of-the-art bulk-heterojunction solar cells [8] reveals that the efficiency is limited by the low opencircuit voltage (Voc) delivered by these devices under illumination. Typically, organic semiconductors with a bandgap of about 2 eV are applied as photoactive materials, but the observed open-circuit voltages are only in the range of 0.5–1 V. There has long been a controversy about the origin of the Voc in conjugated polymer–fullerene solar cells. Following the classical thin-film solar-cell concept, the metal–insulator–metal (MIM) model was applied to bulk-heterojunction devices. In the MIM picture, Voc is simply equal to the work-function difference of the two metal electrodes. The model had to be modified after the observation of the strong influence of the reduction potential of the fullerene on the open-circuit volt