Q1. What are the contributions mentioned in the paper "2‐alkyl-5-thienyl-substituted benzo[1,2‐b:4,5‐b′]dithiophene-based donor molecules for solution-processed organic solar cells" ?
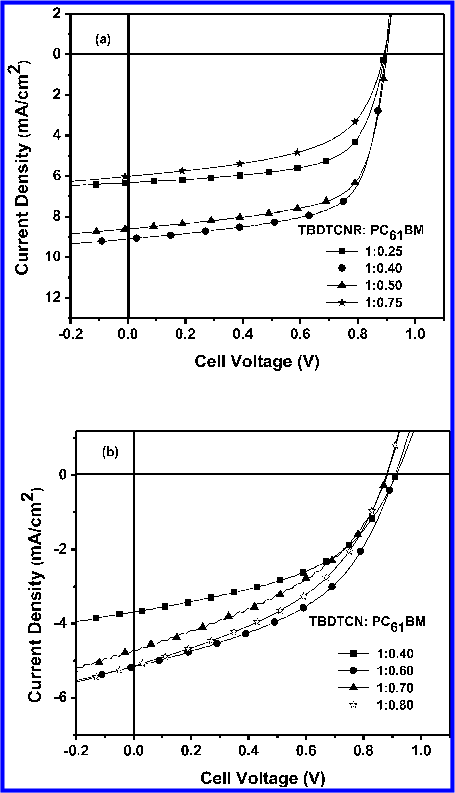
In this study, the authors have strategically designed and convergently synthesized two novel, symmetrical, and linear A− D−A-type π-conjugated donor molecules ( TBDTCNR, TBDTCN ), each containing a planar electron-rich 2-octylthiene-5-ylsubstituted benzodithiophene ( TBDT ) unit as the core, flanked by octylthiophene units and end-capped with electron-deficient cyanoacetate ( CNR ) or dicyanovinyl ( CN ) units. The authors thoroughly characterized both of these materials and investigated the effects of the end groups ( CNR, CN ) on their optical, electrochemical, morphological, and photovoltaic properties. The FFs of these solutionprocessed small-molecule organic solar cells ( SMOSCs ) are outstanding when compared with those recently reported for benzodithiophene ( BDT ) -based SMOSCs, because of the high crystallinity and excellent stacking properties of the TBDT-based compounds.