1
20-Hydroxyecdysone activates the protective arm of the renin angiotensin
system via Mas receptor
René Lafont
1,2
, Sophie Raynal
1
, Maria Serova
1
, Blaise Didry-Barca
1
, Louis Guibout
1
,
Mathilde Latil
1
, Pierre J. Dilda
1*
, Waly Dioh
1
, Stanislas Veillet
1
1
Biophytis, Sorbonne Université – BC9, 4 place Jussieu, 75005 Paris, France.
2
Sorbonne Université, CNRS - Institut de Biologie Paris Seine (BIOSIPE), 75005 Paris,
France
*Corresponding author: Pierre J. Dilda
Email: pierre.dilda@biophytis.com
Running title: 20E, a new Mas receptor activator
Keywords: 20-Hydroxyecdysone (20E), Mas receptor, renin-angiotensin-aldosterone system
(RAAS), ecdysteroid, G protein-coupled receptor (GPCR), estrogen, muscle, myoblast
ABSTRACT
20-Hydroxyecdysone (20E) is a
steroid hormone that plays a key role in
insect development through nuclear
ecdysone receptors (EcRs) and at least one
membrane GPCR receptor (DopEcR) and
displays numerous pharmacological effects
in mammals. However, its mechanism of
action is still debated, involving either an
unidentified GPCR or the estrogen ERβ
receptor. The goal of our study was to better
understand 20E mechanism of action.
A mouse myoblast cell line (C2C12)
and the gene expression of myostatin (a
negative regulator of muscle growth) was
used as a reporter system of anabolic
activity. Experiments using protein-bound
20E established the involvement of a
membrane receptor. 20E-like effects were
also observed with Angiotensin-(1-7), the
endogenous ligand of Mas. Additionally, the
effect on myostatin gene expression was
abolished by Mas receptor knock-down
using small interfering RNA (siRNA) or
pharmacological inhibitors.
17-Estradiol (E2) also inhibited
myostatin gene expression, but protein-
bound E2 was inactive, and E2 activity was
not abolished by angiotensin-(1-7)
antagonists. A mechanism involving
cooperation between Mas receptor and a
membrane-bound palmitoylated estrogen
receptor is proposed.
The possibility to activate the Mas
receptor with a safe steroid molecule is
consistent with the pleiotropic
pharmacological effects of ecdysteroids in
mammals and indeed this mechanism may
explain the close similarity between
angiotensin-(1-7) and 20E effects. Our
findings open a lot of possible therapeutic
developments by stimulating the protective
arm of the renin-angiotensin-aldosterone
system (RAAS) with 20E.
INTRODUCTION
Steroids in animal and plant kingdoms
Steroid hormones are (chole)sterol
derivatives widespread in animals and plants,
where they are involved in the control of plenty
of physiological processes. They include for
example vertebrate sex hormones
(progestagens, estrogens, androgens), insect
moulting hormones (ecdysteroids), as well as
plant growth hormones (brassinosteroids). This
means that the rigid carbon skeleton of sterols
is particularly suitable to generate a very large
number of derivatives, which differ by the
carbon number and/or the position of various
substituents (mainly hydroxyl or keto groups)
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 10, 2020. ; https://doi.org/10.1101/2020.04.08.032607doi: bioRxiv preprint

2
(1). In addition to hormones, sterols give rise to
bile acids/alcohols, initially considered as
emulsifiers facilitating lipid digestion, but
nowadays also known as important signalling
molecules acting on specific receptors (2).
Diversity of steroid mechanisms of action
Our concepts on (steroid) hormone
mechanism of action has evolved. In the
classical scheme, steroid hormones interact
with nuclear receptors and the complex formed
regulates the transcriptional activity of target
genes, which promoters contain specific
sequences (hormone-responsive elements). But
steroids also act at cell membrane level where
they elicit rapid non-transcriptional effects.
Among the identified steroid membrane
receptors, we may mention vertebrate
GPER1/GPR30 (a membrane estrogen receptor
– (3), TGR5 (a bile acid membrane receptor –
(4), MARRS (a calcitriol receptor –(5) or
drosophila DopEcR (a dopamine and ecdysone
membrane receptor – (6). All these receptors
belong to the family of GPCR/7TD receptors.
Moreover, intact or truncated forms or the
steroid nuclear receptors are bound to the
plasma membrane and do not act there as
transcription factors(7,8).
Ecdysteroid effects on vertebrates
We are especially interested by the
pharmacological effects of ecdysteroids on
mammals. Ecdysteroids are a large family of
steroids initially discovered in arthropods
(zooecdysteroids) and later in plants
(phytoecdysteroids) (9). They are present in
many plant species where they can reach
concentrations of up to 2-3 % of the plant dry
weight, and they are expected to protect plants
against phytophagous insects.
With the aim to use these molecules for
crop protection, toxicological studies were
performed on mammals, which unexpectedly
concluded to both their lack of toxicity (oral
LD
50
> 9 g/kg) and their “beneficial” effects,
e.g. anti-diabetic and anabolic properties (10).
Such effects have to be linked with the presence
of large amounts of phytoecdysteroids in
several plants used worldwide by traditional
medicine. At the moment, numerous effects
have been reported, allowing to consider
ecdysteroids as some kind of “universal
remedy”(11). While many effects have been
described on animals (10,12,13), the clinical
evidence for 20E effectiveness in
humans remains limited at the moment
(14-16).
How do ecdysteroids work?
In spite of more than 40 years of
research, the mechanism of action of these
molecules on mammals/humans has not been
elucidated, as only diverging reports are
available for the moment. Several data favour
an action on membranes through a GPCR
receptor(17), whereas other ones suggest the
involvement of a nuclear receptor, the estrogen
receptor ERβ (18,19).
There is in fact no direct evidence for
the binding of 20E to nuclear estrogen (or
androgen) receptors (12,13). The evidence of
ERβ involvement in 20E effect is based on the
use of specific pharmacological activators or
inhibitors of ERs, the former being able to
mimic and the latter to inhibit the effects of
20E on target cells such as osteoblasts (20) or
myoblasts (19). These studies however do not
bring proofs for a direct 20E binding, as ER
receptor could be activated indirectly, and
even in the absence of ligand, e.g. by
phosphorylation (21). Some evidence for a
direct binding was provided by in silico
modelling (22), but this certainely does not
represent a definite proof, as the result may
strongly depend on the model used, and an
opposite conclusion was drawn by Lapenna et
al. (23).
Evidence for membrane effects of 20E
is based on early studies showing the
rapid modulation of several second
messengers (cAMP, cGMP, IP3, DAG, Ca
2+
)
in target cells (24-26) and on the fact that
20E bound to metallic nanoparticles,
preventing its entrance in target cells, is still
active (27). More recently, Gorelick-Feldman
et al. (17) used a pharmacological
approach with various inhibitors (e.g.
pertussis toxin). They concluded that the
membrane 20E receptor belongs to the GPCR
family and proposed a mechanism of
transduction involving an unidentified
GPCR and a membrane calcium channel
(Supporting Fig. S1).
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 10, 2020. ; https://doi.org/10.1101/2020.04.08.032607doi: bioRxiv preprint

3
The above pharmacological arguments
appear strong enough to consider that the cell
membrane is (maybe not exclusively) a site of
action of 20E. The present experiments have
been undertaken in an attempt to identify
the/one GPCR involved in 20E effects, and to
understand its estrogen-like effects using gene
silencing or different pharmacological
approaches.
MATERIALS AND METHODS
Chemicals
Except otherwise mentioned, all the reagents
and chemicals were from Sigma (Saint-Quentin
Fallavier, France). Peptides such as
angiotensin-(1-7), A779 (Asp-Arg-Val-Tyr-Ile-
His-D-Ala) and A1 (Asp-Arg-Val-Tyr-Ile-His-
D-Pro) were custom-prepared by the IBPS
peptide synthesis platform (Sorbonne
University, Paris, France). 20-
Hydroxyecdysone (20E) was obtained from
Chemieliva Pharmaceutical (Chongqing,
China) or from Patheon (Regensburg,
Germany) and had a purity of 96.5-97%.
Preparation of HSA-conjugated 20-
hydroxyecdysone
22-succinyl-20E was prepared according to
Dinan et al.(28). Coupling the 20E derivative to
human serum albumin (HSA) was performed
according to a method provided by Dr J-P
Delbecque (JP Delbecque and M. de Reggi,
personal communication). The 20E-HSA
conjugate (Supporting Fig. S2) was analyzed
by mass spectrometry to determine the number
of 20E molecules coupled to each HSA
molecule. Analyses were performed in a 4700
MALDI TOF/TOF proteomics analyzer
(Applied Biosystems). The 20E conjugate was
studied in linear mode, positive ion mode. Laser
acceleration is set at 20kV and the default laser
fluency was set at 2,000 and modified according
to the signal-to-noise quality. The matrix used
was α-cyano-4-hydrocinnamic Acid (HCCA).
Samples were prepared following the dried-
droplet method, 1 µL of a mixture of 1 µL of
matrix (10 mg/mL) and 1 µL of sample was
spotted and dried with gaseous nitrogen. The
mass shift of HSA around 5 kDa after coupling
indicates that 9 molecules of 20E derivatives are
coupled to each albumin molecule.
Cell culture
The C2C12 mouse myoblast cell line (29) was
purchased from ATCC (CRL-1772). Except
otherwise mentioned, culture media, serum,
antibiotics and supplements were from Life
technologies (Villebon-sur-Yvette, France). All
cultures contained 100 U/mL of penicillin, and
100 μg/mL of streptomycin and are maintained
in a 5% CO
2
, 95% air humidified atmosphere at
37°C. For C2C12 proliferation, cells were
maintained in DMEM medium containing
4.5 g/L glucose supplemented with 10% FBS.
C2C12 cells were maintained at low passage (3-
20 passages) for all experiments to maintain the
differentiation potential of the cultures. Cell
confluency was always kept below or equal to
~80%. For all experiments, cells were first
seeded at 30,000 cells per well in 24-well plates.
To induce differentiation, C2C12 at ~80%
confluency in proliferation medium were
shifted to DMEM medium supplemented with
either 2% FBS or 2% horse serum.
Protein synthesis (
3
H-leucine incorporation)
C2C12 cells were grown on 24-well
plates at a density of 30,000 cells/well in 0.5 mL
of growth medium (DMEM 4.5 g/L glucose
supplemented with 10% fetal bovine serum).
Twenty-four hours after plating, the
differentiation induction into multinucleated
myotubes was carried out in DMEM 4.5 g/L
glucose containing 2% fetal bovine serum.
After 5 days, cells were pre-incubated in Krebs
medium 1 h at 37°C before being incubated in
DMEM media without serum for 2.5 h in the
presence of radiolabeled leucine (5 µCi/mL)
and DMSO (control condition) or Insulin
Growth Factor (IGF-1, 100 ng/mL) or 20E (0.1
– 0.5 – 1 – 5 -10 µM). At the end of incubation,
supernatants were discarded and cells were
lysed in 0.1 N NaOH for 30 min. The cell
soluble fraction-associated radioactivity was
then counted using Wallac Microbeta 1450-021
TriLux Luminometer Liquid Scintillation
Counter (Wallac EG&G, Gaithersburg, MD,
USA) and protein quantification was performed
using the colorimetric Lowry method.
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 10, 2020. ; https://doi.org/10.1101/2020.04.08.032607doi: bioRxiv preprint

4
Myostatin and MAS gene expression assays
Cells were plated at a density of 30,000
cells per well in 24-well plates and were grown
overnight in 5 % CO
2
at 37°C. On day 5 of
differentiation, treatments were carried out for
6 h. At the end of incubation, RNAs were
extracted and purified using the RNAzol
(Eurobio, Les Ulis, France). RNAs were
converted into cDNAs with High-Capacity
cDNA Reverse Transcription Kit (Applied
Biosystems, ThermoFisher, Villebon-sur-
Yvette, France) before performing a
quantitative PCR using iTaq SybrGreen
(Biorad, Marnes-la Coquette, France).
Q-RT-PCRs were then performed using a
7900HT Fast Real-Time PCR detection system
(Applied Biosystems) and standard qPCR
program (1 cycle 95°C 15 min followed by 40
cycles 95°C 15s and 60°C 1 min). QRT-PCR
master mix contained the 100 ng cDNA
samples and a set of primers at final
concentration of 200 nM designed into two
different exons and described below. The
quality of RNA was checked using the
nanodrop™ technology (ThermoFisher) when
necessary.
The relative differences in gene expression
levels between treatments were expressed as
increases or decreases in cycle time [Ct]
numbers compared to the control group where
the [Ct] value of each gene was normalized to
the beta actin gene or hypoxanthine guanine
phosphoribosyl transferase (HPRT) gene.
Results of gene expression were expressed in 2
-
∆∆CT
after normalization with house-keeping
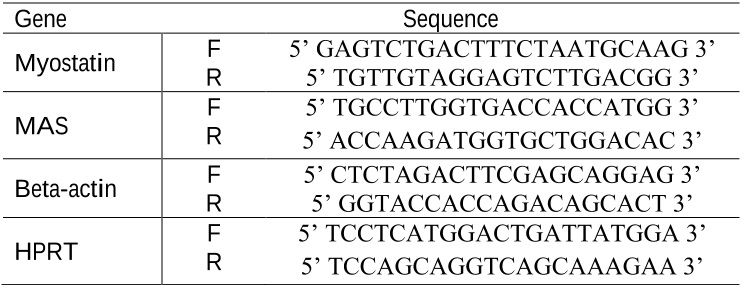
genes. Primer sequences used are described in
Table 1.
SiRNA MAS assay
Cells were plated at a density of 10,000
cells per well in 24-well plates. After 3 days of
differentiation, cells were transfected either
with scramble SiRNA (10 nM) or MAS-1
SiRNA (10 nM) according to manufacturer’s
instructions (Origene Technologies, Rockville,
MD, USA). Two days after transfection,
myotubes were treated with either DMSO or
IGF-1 or 20E or angiotensin-(1-7) for 6 h. At
the end of incubation, RNA was extracted and
analyzed by QRT-PCR as described above.
Binding studies
The affinity of 20E for human nuclear
steroid receptors such as androgen receptor
(AR), estrogen receptors alpha and beta (ERα,
ERβ) and glucocorticoid receptor (GR) was
determined by radioligand binding assays
(CEREP/Eurofins). The selective ligands [
3
H]-
methyltrienolone, [
3
H]-estradiol, [
3
H]-
dexamethasone were employed on cells
expressing either human endogenous or
recombinant AR, ERα or β, or GR, respectively.
20E was used at concentrations up to 100 μM as
a potential competitor. Inhibition of control
specific binding was determined, and IC
50
and
K
i
were calculated when possible. Additionally,
a receptor screen was carried out on 45 GPCR
and 5 nuclear receptors at a fixed concentration
of 20E (10 μM). Radioligand binding assays
were performed according to manufacturer
instructions employing
3
H- or
125
I-labelled
specific ligands of each receptor
(SafetyScreen87 Panel, Panlabs, Taipei,
Taiwan).
Statistical analyses
Statistical analysis was performed
using Graph Pad Prism® Software. Anova
followed by a Dunnett t-test or a Kruskal Wallis
followed by a Dunn’s test when the variances
significantly differed have been performed. To
evaluate the significance of differences between
two groups, the choice of parametric Student t-
test or non-parametric Mann-Whitney test was
based on the normality or non-normality of data
distribution, respectively (D’Agostino &
Pearson test). The results are considered
significant at p-value <0.05 (*), <0.01(**),
<0.001 (***).
RESULTS
20-Hydroxyecdysone stimulates muscle
anabolism
20-Hydroxyecdysone (20E) effects were
investigated on pre-established murine
myotubes (following 6 days of differentiation).
An anabolic effect was investigated through de
novo protein synthesis assay. A dose-dependent
increase in protein synthesis was observed in
response to 20E treatment of C2C12 myotubes
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 10, 2020. ; https://doi.org/10.1101/2020.04.08.032607doi: bioRxiv preprint

5
versus untreated conditions (Fig. 1A). IGF-1
(100 ng/mL), employed as positive control (30)
displayed, as expected, an improvement in [
3
H]-
Leu incorporation (+20%, p < 0.001). 20E
effect was significant from 0.5 μM to 5 μM. The
maximal effect (+ 27 %; p < 0.001) was
measured with 5 μM of 20E, while a treatment
with a higher concentration of 20E (10 μM)
appeared to be notably less efficient (+11%, ns)
than the previous concentration tested.
Myostatin is a major autocrine regulator that
inhibits muscle growth in mammals. The
myostatin transcript bioassay was developed
and standardized in order to assess ecdysteroid
activity (31). IGF-1 (100 ng/mL) used as a
positive control demonstrated a significant
inhibition of myostatin gene expression (57% of
untreated control cells, p<0.001, Fig. 1B). A
dose-dependent and partial inhibition of
myostatin gene expression was observed in
response to 20E treatment at concentrations
comprised between 0.001 and 10 µM. This
inhibition reached significance from 0.5 µM
20E (Fig. 1B). The inhibition of myostatin gene
expression was then employed as a readout for
20E activity.
20-Hydroxyecdysone acts on cell membranes
In order to confirm that 20E acts primarily on
cell membrane, or if it needs to penetrate into
the cell to exert its effects, a membrane-
impermeable derivative of 20E was produced
(Supporting Fig. S2). We compared the effects
of free 20E and its 22S-HSA conjugate on
myostatin gene expression at the concentration
of 10 µM 20E-equivalents (Fig. 2). We
observed that the membrane-impermeable 20E-
derivative retained an activity similar to that of
free 20E. This results demonstrates that the
presence of a bulky protein does not prevent
20E activity and is an additional argument for
the interaction of 20E with a membrane
receptor, as proposed by Gorelick-Feldman et
al. (2010).
20-Hydroxyecdysone acts via a GPCR-type
receptor
The GPCR hypothesis is based on the inhibition
of 20E effects by pertussis toxin, but there are
still numerous possible GPCR candidates. We
selected a set of GPCR receptors based on
available literature and using different criteria
corresponding to well-established effects of
20E: (i) involvement in the control of muscle
cells activity and glycaemia/insulin sensitivity,
and (ii) ability to reduce fat mass gain in high-
fat fed animals (32,33). A set of GPCRs was thus
selected including TGR5 (bile acids receptor -
(4), GPER/GPR30 (estradiol, aldosterone
receptors -(3,6), LPA1 (lysophosphatidic acids
receptor -(34), APJ (apelin receptor -(35),
OXTR (oxytocin receptor -(36), AVPR1
(vasopressin receptor -(37), MrgD (alamandine
receptor - (38), MARRS (vitamin D3 receptor -
(5) and Mas (angiotensin-(1-7) receptor -(39).
The possible interaction of 20E with those
receptors was assayed using different
approaches according to available
methodologies: (1) in silico binding when 3D
structures were available, (2) in vitro direct
binding studies by competition with a
radioactive natural ligand, (3) comparison of the
effects of agonists with those of 20E on C2C12
cells or (4) effect of known antagonists on the
response of C2C12 cells to 20E. Using this
approach, the only receptor which gave positive
data was Mas, the receptor of angiotensin-(1-7).
20-Hydroxyecdysone and Angiotensin-(1-7)
act via Mas receptor activation
Using the myostatin gene expression assay, a
pharmacological approach was emloyed to
compare the effects of the endogenous Mas
receptor agonist (angiotensin-(1-7)) with those
of 20E on C2C12 cells in the presence and
absence of known antagonists.
Angiotensin-(1-7) (Ang 1-7, Supporting Fig.
S3) as well as 20E (Fig. 1B) partially inhibits
myostatin gene expression in a dose-dependent
manner. As expected, this inhibition by Ang 1-
7 (10 µM) was totally abolished by specific Ang
1-7 antagonists (A1 or A779, 10 µM) (Fig. 3A).
Interestingly, 20E inhibitory effects on
myostatin gene expression was also fully
reverted by the same antagonists (Fig. 3A)
suggesting that inhibition of myostatin gene
expression by 20E (or Ang 1-7) is mediated by
the receptor of Ang 1-7. In contrast, the effect
of IGF-1, which acts through its own receptor
(insulin-like growth factor 1 receptor; IGF1R)
remains unchanged in the presence or in the
(which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission.
The copyright holder for this preprintthis version posted April 10, 2020. ; https://doi.org/10.1101/2020.04.08.032607doi: bioRxiv preprint

![Figure 1: Effects of 20E on protein synthesis and Myostatin gene expression in C2C12 cells. (A) Experiments displaying 20E effects on protein synthesis in differentiated myotubes detected by [3H]-leucin incorporation. Results are shown as means ± SEM, ***p < 0.001, **p < 0.01, *p < 0.05 vs untreated control (Kruskal-Wallis followed by a Dunn’s test). (B) C2C12 mouse myoblasts were differentiated for 6 days into myotubes. They were then treated for 6 hours with concentrations of 20E ranging from 0.001 to 10 μM. Myostatin gene expression was detected by qRT-PCR. Results are shown as means ± SEM with ***p<0.001, **p<0.01, *p<0.05 vs untreated control (one-way ANOVA with Dunnett’s test compared to untreated control).](/figures/figure-1-effects-of-20e-on-protein-synthesis-and-myostatin-gikt80ej.png)