




Did you find this useful? Give us your feedback










8 citations
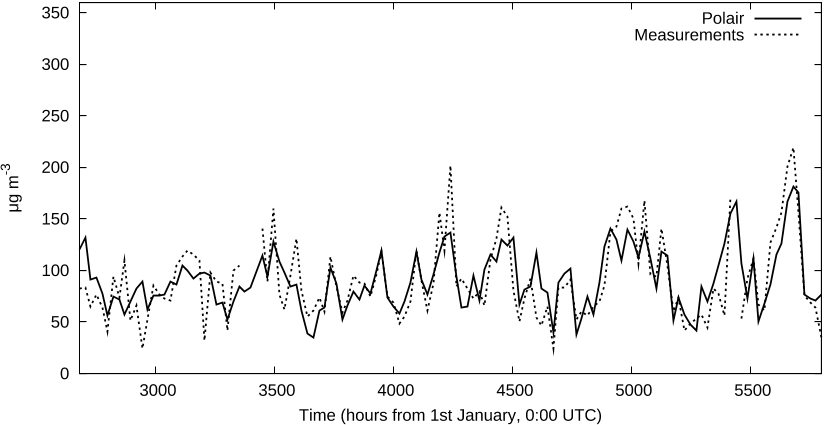
...A key tool for part of these functionalities is the adjoint model of Polair3D [Mallet and Sportisse, 2004]....
[...]
7 citations
...It must be noted that the increasing development of non linear chemistry transport models (Mallet and Sportisse, 2004) is a field of application of inverse techniques, in the hypothesis of linear perturbations....
[...]
7 citations
...Additional sources i S ( , emissions) and losses i L ( , wet and dry deposition) are included (Mallet and Sportisse, 2004)....
[...]
...Additional sources iS( , emissions) and losses iL( , wet and dry deposition) are included (Mallet and Sportisse, 2004)....
[...]
7 citations
5 citations
2,357 citations
2,230 citations
...Dry gaseous deposition Di is computed as in Wesely (1989) or as in Baer et al.15 (1992), and required land use coverage data may be provided by the USGS data base (http://edcdaac.usgs.gov/glcc/glcc.html)....
[...]
...Meteorological data could be processed in a better way, deposition velocities should be computed thanks to Wesely (1989) or Baer et al. (1992) which are now available in the code, biogenic emissions should improve results specially outside the plume....
[...]
...Deposition velocities are computed according to15 Wesely (1989)....
[...]
1,086 citations
...…based on Petersen (1995);10 – Aerosols: modal approximation for inorganic aerosols; – Photochemistry (for ozone): several mechanisms are available among which RADM 2 (Stockwell et al., 1990), RACM (Stockwell et al., 1997), EURORADM (Schell, 2000), MOCA (Aumont, 1994), CBM IV (Gery et al., 1989)....
[...]
...– Aerosols: modal approximation for inorganic aerosols; – Photochemistry (for ozone): several mechanisms are available among which RADM 2 (Stockwell et al., 1990), RACM (Stockwell et al....
[...]
...The main chemical mechanism was RADM 2 (see Stockwell et al., 1990), with 6115 species and 157 reactions....
[...]
960 citations
...…based on Petersen (1995);10 – Aerosols: modal approximation for inorganic aerosols; – Photochemistry (for ozone): several mechanisms are available among which RADM 2 (Stockwell et al., 1990), RACM (Stockwell et al., 1997), EURORADM (Schell, 2000), MOCA (Aumont, 1994), CBM IV (Gery et al., 1989)....
[...]
...For the time being, Polair has several chemical mechanisms: – Mercury chemistry: simplified mechanism based on Petersen (1995);10 – Aerosols: modal approximation for inorganic aerosols; – Photochemistry (for ozone): several mechanisms are available among which RADM 2 (Stockwell et al., 1990), RACM (Stockwell et al., 1997), EURORADM (Schell, 2000), MOCA (Aumont, 1994), CBM IV (Gery et al., 1989)....
[...]
..., 1990), RACM (Stockwell et al., 1997), EURORADM (Schell, 2000), MOCA (Aumont, 1994), CBM IV (Gery et al....
[...]
...One may use RACM instead....
[...]
...The chemical mechanism is RACM....
[...]
928 citations
The main10 future works will be devoted to aerosol modeling and air-quality ensemble forecast.