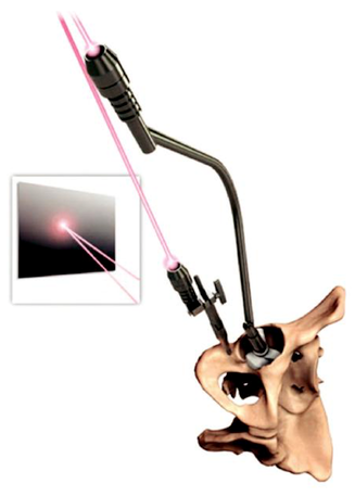
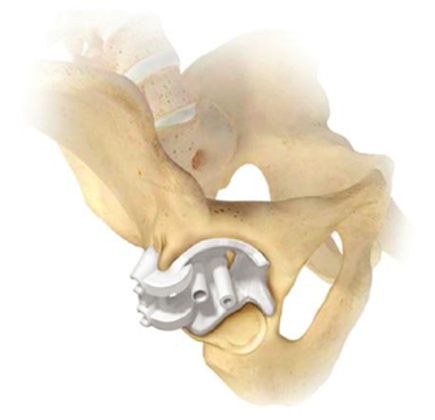
1
3D-printed Patient-specific Guides for Hip Arthroplasty
Johann Henckel, MD Thomas J. Holme, MD Warwick Radford, MD John A. Skinner, MD
Alister J. Hart, MD
Abstract
Surgeons and engineers constantly search for methods to improve the surgical positioning of
implants used for joint arthroplasty. Rapid prototyping is being used to develop patient-
specific instrumentation (PSI) and has already been successfully translated into large-scale
clinical use for knee arthroplasty. PSI has been used in shoulder arthroplasty; however, it is
not yet known whether PSI provides improved accuracy and outcomes compared with
conventional methods in either shoulder arthroplasty or knee arthroplasty. In the hip, PSI has
been limited to the positioning of custom-manufactured implants and a small number of
surgeons testing the emerging solutions from different manufacturers. Early results indicate
consistent accurate positioning of implants with the use of PSI in hip arthroplasty but with
added costs and uncertain effect on clinical outcomes.
Introduction
The clinical function of a hip ar- throplasty depends on surgical, implant, and patient factors.
Surgical factors include component position- ing,
1
which is a modifiable risk factor and is
known to contribute to patient dissatisfaction,
2
dislocation, wear, impingement, decreased
range of motion, and leg length discrepancy.
3-7
Acetabular loosening occurs in up to 50% of
cases after hip arthroplasty.
8
Computer-aided surgery and robot- ics have been used to improve the accuracy of component
positioning in hip arthroplasty but have had limited uptake because of high costs, increased
operating times, and other logistic issues.
9,10
Reports of image-guided hip arthroplasty are
encouraging, but data from the National Joint Registry show that conventional instrumented
sur- gery remains the standard treatment.
11
More recently, patient-specific in- strumentation (PSI) has been developed to guide the
positioning of components during hip arthroplasty. This technique uses imaging techniques
such as CT and MRI to plan surgery in a virtual three- dimensional (3D) environment. The
surgeon can then plan prosthesis ori- entation and position in relation to a chosen standard
frame of reference and execute the plan using simple intra- operative patient-specific guides.
Here, we present the currently commercially available guides and summarize the relevant
literature regarding PSI for hip arthroplasty in order to provide an overview of PSI and
describe the evidence regarding its use in clinical practice.
Rapid prototyping was initially de- veloped in the mid 1980s for the production of toys and
household machinery and has been used for a wide variety of medical and nonmedical
purposes since that time.
12
PSI has evolved out of this and has been taken up in numerous
fields of surgery. The first reported use in orthopaedics was in the spine in 1998 for
producing guides to aid in the placement of

2
pedicle screws
13
and has since spread to include guides for shoulder, hip, knee, and ankle
elective and trauma surgery.
PSI has been increasingly commer- cially used in total knee arthroplasty to produce custom-
made pinning and cutting guides;
14
however, recent lit- erature has questioned whether the
use of PSI improves the accuracy of im- plant alignment or clinical outcomes compared with
standard instrumenta- tion.
15,16
Studies using PSI for shoulder and ankle arthroplasty show
promis- ing early results in terms of the accu- racy of implant placement; however,
commercial applications have not been developed and no study has demon- strated improved
clinical outcomes in shoulder or ankle arthroplasty.
17-21
Clinical Application in Hip Arthroplasty
PSI is being used in hip arthroplasty to improve the accuracy of acetabular and femoral
component position- ing.
22-24
Acetabular guidance systems aim to optimize the cup size,
implant medialization, anteversion, and incli- nation. Femoral guidance systems aim to
optimize the stem size and positioning, offset, leg length (height of neck cut), and stem
version. Four commercial systems are currently available internationally (Table 1 and Figures
1–3). In addition, a number of patents have been registered on devices that have been
modified with the intent to improve on current designs. The Signature Hip (Zimmer Biomet),
OPS (Corin Group), and MyHip (Medacta) are currently available for use in the United States
and Europe, whereas the Hip Plan (Symbios) is currently avail- able for use only in Europe.
All systems required preoperative imaging with either CT or MRI to cre- ate the patient-
specific model and tem- plate the guides and implants required. CT provides well-defined
bony anat- omy with low levels of artifact; however, it has limitations in demonstrating soft
tissue. The commercial systems available use a low-dose radiation protocol, delivering
slightly higher radiation exposure compared with conventional radiographs, with a 2011
study reporting a scan time of 11 minutes and a direct per-patient cost of V52.80 ($54.94).
25
Although MRI is better for visualizing detail within the soft tissues, it offers less well-defined
soft tissue–bone boundaries compared with CT. For example, it is difficult to differentiate
osteophytes from soft tisues on MRI. Neither imaging modaility has been proved to be su-
perior for PSI in total hip arthroplasty (THA), and which is used depends on the system
chosen (Table 1). It is as yet uncertain which is the optimal imag- ing modality for creation of
PSI in total hip replacement and both are currently available depending on the commercial
system chosen (Table 1).
PSI requires 3D preoperative plan- ning and therefore incorporates the known and obvious
advantages of 3D compared with 2D planning.
26
Three- dimensional reconstruction software
is used to create a 3D computer model. This 3D model is used to virtually plan the
positioning and sizing of the prostheses. The frames of reference and target
positioning/orientation of the implants can be tailored to the surgeon’s preference. Two of the
commercially available models include kinematic simulation to take into account the
influence of pelvic tilt and assess the potential functional effect of a chosen implant position
in terms of bony and implant impingement (ie, MyHip, OPS). The guide is de- signed to fit
and complement the patient’s native anatomy using bony and soft-tissue landmarks on the CT

3
or MRI scan. The physical 3D guide is created and produced using a number of methods,
including selective laser sintering and additive materials manufacturing (3D printing) and
sterilized before delivery to the surgeon’s center. The whole process can take as little as 3
weeks from start to finish;
27
however, there is typically a lead time of approximately 6 to 8
weeks while the PSI guide is created.
Of the commercially available hip PSI systems, all four offer guides for the acetabular
component orientation, but only two of the systems offer a guide for femoral component
orientation (ie, MyHip, OPS). The surgical technique varies between products, with anterior,
posterior, and lateral surgical approaches available depending on the system chosen (Table
1).
The depth of acetabular reaming and therefore planning for accurate medialization of the
implant are still determined by hand and clinical judgment, although future models may
include a guidance feature. Pins can be used to guide alignment of the reamer if desired.
Preoperative 3D printed acetabular models are avail- able to help with understanding the
patient’s unique anatomy and how the guide/implant should fit before attempting insertion
into the patient (Figure 4).
Insertion guides come in two broad categories: constrained and nonconstrained, depending on
whether the guide simply shows the correct direction of implantation or physically guides
insertion. Accurate insertion of the guides depends on bony (ie, acetabular dome, rim, notch)
and soft- tissue (ie, transverse acetabular ligament) landmarks, which are used to place a
custom jig into the acetabulum. As with conventionally guided surgery, the surgical exposure
is important in getting this step right. The positioning of the implants is then guided by either
pins or lasers. All require care when preparing the surgical site, particularly with soft-tissue
removal so that land- marks used in planning are not removed; this is of particular impor-
tance in cases in which osteophytes are removed.
28
Only the MyHip and OPS systems include a femoral component guide. Posterior and anterior
cutting guides are offered so that the surgeon can choose the guide best suited to the preferred
surgical approach. This guides the neck cut level and angle but does not guide stem version.
Does Patient-specific Instrumentation Improve the Accuracy of Cup Orientation?
Buller et al
29
undertook a dry bone simulation study, with seven sur- geons performing THA
performed with standard instrumentation, fol- lowed by PSI-guided THA. The surgical goal
was to accurately place the acetabular implant in 22 of anteversion and 40 of inclination
as per a preoperative plan. In the standard instrumentation group, six of seven acetabular
components were placed in an unacceptable position with regard to inclination and version
(using “safe zones” of 15 6 10 for anteversion and 40 6 10 for inclination). In the PSI
group, three of seven acetabu- lar components were malpositioned with regard to version, and
none was malpositioned with regard to inclination.
Shandiz et al
30
undertook a cadaver study in which they implanted PSI- guided acetabular
components into 12 hips. Preoperative CT scans were used in surgical planning, and post-
operative CT scans were used to measure implant positioning and ori- entation. This
demonstrated the ability to accurately position the component using the PSI guide to within
2.5 of planned, with a maximum deviation from planned of 4.7.

4
Schwarzkopf et al
31
undertook a cadaver study of 14 acetabuli using the Bullseye Hip
Replacement In- struments (Bullseye Hip Replace- ment) PSI for acetabular preparation and
cup placement. CT and MRI were used preoperatively to deter- mine the surgical plan and
create the PSI. Postoperative CT scans were used to demonstrate the accuracy of the
placement of implants. The ace- tabular cup inclination and ante- version angles were within
the target range, and all implanted sizes matched the preoperative surgical planned implant
size.
In a prospective randomized con- trolled trial, Small et al
32
compared 18 patients undergoing
THA with conventional instrumentation and 18 patients undergoing THA with PSI. Pre- and
post-operative CT scans were used to evaluate planned versus actual results. Results dem-
onstrated a statistically significant difference in version of the acetabu- lar component
between standard instrumentation and PSI (P = 0.018; mean difference from planned versus
actual anteversion of 26.9 6 8.9 for standard instrumentation and 20.2 6 6.9 for PSI
cases). The difference for inclination was not statistically significant (mean differ- ence in
abduction from planned versus actual of 1.3 6 9.1 for standard instrumentation and 22.0
6 7.3 for PSI cases).
In a nonrandomized prospective study, Hananouchi et al
33
compared 38 patients undergoing
THA with traditional instrumentation to 31 patients undergoing THA with PSI. The planned
versus actual position of the acetabular component was eval- uated with pre- and
postoperative CT scans, determining the incidence of outliers beyond 10 from planned
placement in each group. The study authors reported that the use of PSI reduced the number
of outliers compared with the use of standard instrumentation (zero versus 23.7%,
respectively). This trend was statis- tically significant for mean inclina- tion (P = 0.01) but
not for mean anteversion (P = 0.08).
In a prospective study, Spencer- Gardner et al
27
treated 100 patients using the OPS PSI for
cup placement. They used 3D CT planning software to preoperatively choose the optimal
acetabular inclination and version for each patient. A posterolateral surgical approach was
used for each patient, and the PSI laser guidance system was used for the accurate placement
of the acetabular implants. The accuracy of implant placement was evaluated using 3D CT to
compare the actual position with the preoperative plan. They demonstrated accurate place-
ment to within 5 in 54% of patients and to within 10 in 91% of patients. These results are
comparable with robotic and computer-navigated techniques and superior to reported
freehand techniques.
Ito et al
34
prospectively evaluated 10 patients in whom PSI was used for femoral stem
placement. CT scans were used for preoperative planning. In all patients, noncemented
implants were placed via the posterior surgical approach. Postoperative CT scans were used
to demonstrate the accuracy of the placement of im- plants. Results demonstrated a mean
accuracy of stem tilt of 2.1 6 4.1, varus/valgus of 1.0 6 0.7, and anteversion of 4.7 6
1.2.
Some pitfalls to accurate cup implantation have been highlighted, including errors made
during the impaction process of cup implantation. Extra care should be taken at this stage. In
addition, the presence of osteo- phytes can sometimes pose a challenge to the accurate
placement of the PSI guide and must be taken into account when executing the surgical
plan.
28

5
PSI for the placement of the femoral component of hip resurfacing has also been investigated,
with promis- ing early results in terms of stem-shaft angle and version.
35
Does the Use of Patient- specific Instrumentation Affect the Duration of Surgery?
Hananouchi et al
33
reported a mean surgical time of 106.1 minutes with PSI, compared with
116.3 minutes with standard instrumentation. This difference was not significant. In the PSI
group, the mean time to use the surgical guide was 3.6 minutes.
Spencer-Gardner et al
27
found that the total operating time increases by 3 to 5 minutes with
use of PSI. This compares to time increases in navi- gated THA of 8 to 58 minutes. Ito et al
34
used PSI for femoral component insertion and demonstrated a mean surgical time of 111
minutes. Small et al
32
demonstrated a mean surgical time of 95 minutes for the PSI group
versus 88 minutes for the standard instrumentation group; this trend was not found to be
statistically significant.
Is Patient-specific Instrumentation Useful in Cases With Massive Bone Defects and
Abnormal Anatomy?
Substantial deformity and insufficient bone structure or quality are contra- indications for the
use of currently available PSI guides for hip arthro- plasty. The dynamic modeling used
preoperatively with PSI requires nor- mal anatomy and sites of rigid attachment for guiding
instruments intraoperatively. Further develop- ments in the design of these guides may result
in the ability to use these systems in patients with more severe deformity.
How Much Does Patient- Specific Instrumentation Add to the Operating Costs?
Most surgeons would use the cur- rently available commercial PSIs, which in our experience
add ap- proximately $371 to each surgical case. For individual hospital pro- duction of PSI,
in 2010 Hananouchi et al
33
reported startup costs of up to $150,000, including $15,000 to
$30,000 for software and $120,000 for the rapid prototyping machine itself, along with a
material cost per case of $50 to $100. The long-term effect of these guides in reducing the
need for future medical treatment for early failures of poorly positioned implants is not yet
known, but the potential financial implications of this may well out- weigh the added initial
costs.
Does the Use of Patient- Specific Instrumentation Have Any Other Intraoperative
Effects?
Blood Loss: Hananouchi et al
33
demonstrated a mean blood loss of 655.9 mL for PSI versus
683.9 mL for standard instrumentation; this difference was not statistically significant. Ito et
al
34
reported a mean estimated blood loss of 356 mL using PSI for the femoral component.
Small et al
32
demon- strated mean estimated blood loss of 200 mL in the PSI group
compared with 150 mL in the traditional in- strumented group; this trend was not found to be
statistically significant.
Complications: Spencer-Gardner et al
27
reported one complication in a series of 100
patients in whom PSI was used for cup placement—a fractured ceramic liner due to