




Did you find this useful? Give us your feedback
![Figure 1. Structure of DMAMQ (2-[(dimethylamino)methyl]-8-hydroxyquinoline).](/figures/figure-1-structure-of-dmamq-2-dimethylamino-methyl-8-1jw2kicu.png)









64 citations
...Indeed, studies using a homologous terdentate 8HQ resulted in a significant increase in pGSK 3β only at cytotoxic concentrations (Haigh et al., 2016)....
[...]
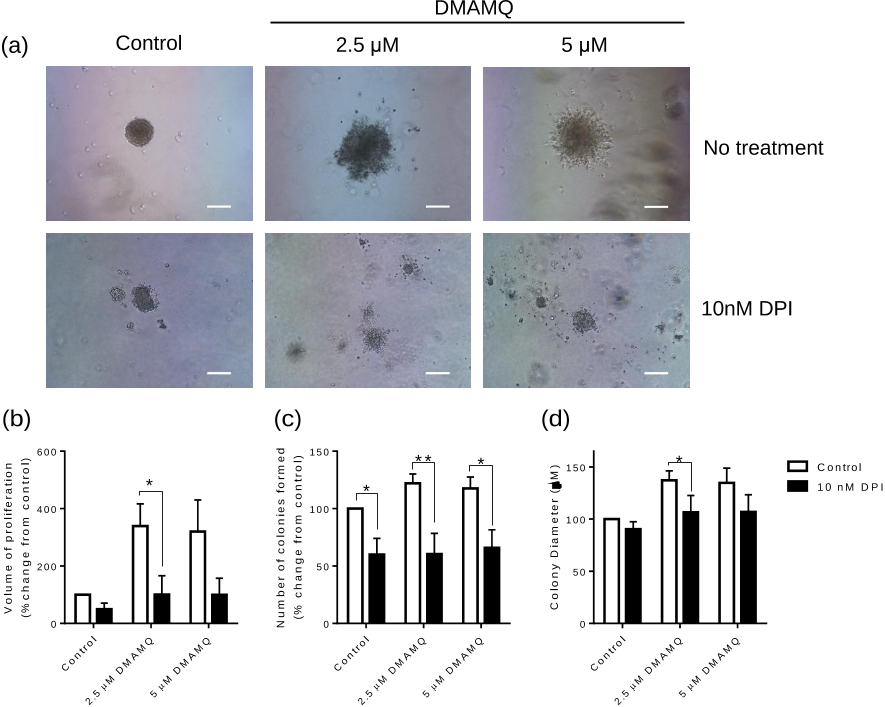
...…in the presence of the biological reductant such as ascorbate, the dominant ternary metal complexes can produce as many hydroxyl radicals as Cu(Aβ1−x) in vitro (Mital et al., 2016) and ROS production can be observed following addition of such 8HQs to neural stem cell cultures (Haigh et al., 2016)....
[...]
..., 2016) and ROS production can be observed following addition of such 8HQs to neural stem cell cultures (Haigh et al., 2016)....
[...]
59 citations
52 citations
...Interestingly, the generation of ROS can be detected by adding such ligands to the culture of neural stem cells [324], in contrast to the founding principle of therapeutic chelation therapy [325]....
[...]
35 citations
31 citations
95 citations
92 citations
...A ‘cellular redox cycle’ has been shown to occur within the cell cycle and is thought to control cycle phase progression [41, 42]....
[...]
88 citations
...Redox signalling pathways have been linked with NSC growth and an increased ROS burst has been linked with induction of proliferation [38,39,40]....
[...]
77 citations
74 citations
In particular, the potential benefits of replenishing damaged brain tissue must be balanced against the possibility of overstimulating neurogenesis and the propensity for undesirable “ off-target ” effects.