RESEARCH ARTICLE
A Bayesian phase 2 model based adaptive
design to optimise antivenom dosing:
Application to a dose-finding trial for a novel
Russell’s viper antivenom in Myanmar
James A. Watson
ID
1,2☯
*, Thomas Lamb
ID
2,3☯
, Jane Holmes
ID
4
, David A. Warrell
ID
2
, Khin
Thida Thwin
5
, Zaw Lynn Aung
5
, Min Zaw Oo
6
, Myat Thet Nwe
ID
3
, Frank Smithuis
2,3
,
Elizabeth A. Ashley
ID
2,3,7
1 Mahidol-Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University,
Bangkok, Thailand, 2 Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine,
University of Oxford, Oxford, United Kingdom, 3 Myanmar-Oxford Clinical Research Unit, Yangon, Myanmar,
4 Centre for Statistics in Medicine, Nuffield Department of Medicine, University of Oxford, Oxford, United
Kingdom, 5 University of Medicine 1, Yangon, Myanmar, 6 University of Medicine 2, Yangon, Myanmar,
7 Lao-Oxford-Mahosot Hospital Wellcome Trust Research Unit, Vientiane, Laos
☯ These authors contributed equally to this work.
* jwatowatson@gmail.com
Abstract
For most antivenoms there is little information from clinical studies to infer the relationship
between dose and efficacy or dose and toxicity. Antivenom dose-finding studies usually
recruit too few patients (e.g. fewer than 20) relative to clinically significant event rates
(e.g. 5%). Model based adaptive dose-finding studies make efficient use of accrued
patient data by using information across dosing levels, and converge rapidly to the con-
textually defined ‘optimal dose’. Adequate sample sizes for adaptive dose-finding trials
can be determined by simulation. We propose a model based, Bayesian phase 2 type,
adaptive clinical trial design for the characterisation of optimal initial antivenom doses in
contexts where both efficacy and toxicity are measured as binary endpoints. This design
is illustrated in the context of dose-finding for Daboia siamensis (Eastern Russell’s viper)
envenoming in Myanmar. The design formalises the optimal initial dose of antivenom as
the dose closest to that giving a pre-specified desired efficacy, but resulting in less than a
pre-specified maximum toxicity. For Daboia siamensis envenoming, efficacy is defined as
the restoration of blood coagulability within six hours, and toxicity is defined as anaphy-
laxis. Comprehensive simulation studies compared the expected behaviour of the model
based design to a simpler rule based design (a modified ‘3+3’ design). The model based
design can identify an optimal dose after fewer patients relative to the rule based design.
Open source code for the simulations is made available in order to determine adequate
sample sizes for future adaptive snakebite trials. Antivenom dose-finding trials would ben-
efit from using standard model based adaptive designs. Dose-finding trials where rare
events (e.g. 5% occurrence) are of clinical importance necessitate larger sample sizes
than current practice. We will apply the model based design to determine a safe and
PLOS NEGLECTED TROPICAL DISEASES
PLOS Neglected Tropical Diseases | https://doi.org/10.1371/journal.pntd.0008109 November 16, 2020 1 / 18
a1111111111
a1111111111
a1111111111
a1111111111
a1111111111
OPEN ACCESS
Citation: Watson JA, Lamb T, Holmes J, Warrell
DA, Thwin KT, Aung ZL, et al. (2020) A Bayesian
phase 2 model based adaptive design to optimise
antivenom dosing: Application to a dose-finding
trial for a novel Russell’s viper antivenom in
Myanmar. PLoS Negl Trop Dis 14(11): e0008109.
https://doi.org/10.1371/journal.pntd.0008109
Editor: Ulrich Kuch, Goethe University, GERMANY
Received: February 2, 2020
Accepted: October 10, 2020
Published: November 16, 2020
Peer Review History: PLOS recognizes the
benefits of transparency in the peer review
process; therefore, we enable the publication of
all of the content of peer review and author
responses alongside final, published articles. The
editorial history of this article is available here:
https://doi.org/10.1371/journal.pntd.0008109
Copyright: © 2020 Watson et al. This is an open
access article distributed under the terms of the
Creative Commons Attribution License, which
permits unrestricted use, distribution, and
reproduction in any medium, provided the original
author and source are credited.
Data Availability Statement: The results in the
manuscript are from simulated data. All underlying
code can be found at: https://github.com/
jwatowatson/AdaptiveAntivenomDesign.

efficacious dose for a novel lyophilised antivenom to treat Daboia siamensis envenoming
in Myanmar.
Author summary
Snakebite envenoming is one of the most neglected tropical diseases considering its bur-
den of mortality and morbidity. Antivenoms are the only known effective treatment for
snake-bite envenoming but are frequently responsible for high rates of adverse reactions.
Clinical development of antivenoms rarely follows the iterative phases of clinical develop-
ment applied to other drugs. Dosing is typically based on pre-clinical testing. Here we
propose a Bayesian model based adaptive design for phase 2 clinical trials aiming to deter-
mine the optimal dose of antivenom needed for treatment of snakebite envenoming. Opti-
mality is defined using safety and efficacy thresholds contextual to the study. This design
can be applied to all antivenoms which have binary efficacy and toxicity endpoints. Our
design formally specifies a desired efficacy and a maximum tolerated toxicity. We use sim-
ulation studies to characterise adequate sample sizes to determine an approximately opti-
mal dose under different scenarios. The simulation studies highlight the advantages of a
model based design over simpler rule based alternatives. This design will be used to deter-
mine an effective and safe dose of the new lyophilised viper antivenom currently in use to
treat Russell’s viper envenoming in Myanmar.
Introduction
Snake-bite envenoming (SBE) was re-categorized as a priority neglected tropical disease by the
World Health Organization (WHO) in 2017 [1, 2]. Worldwide, there are as many as 2.7 mil-
lion people affected by SBE resulting in an estimated 81,000 to 138,000 deaths per year [3–5],
the burden of which disproportionately affects the poorest communities [5–7]. Antivenom is
considered to be one of the most cost effective health interventions [8]. Despite this, due to
challenges in manufacture, reliance on cold chain for transport and storage, and the geograph-
ically remote location of most envenomed patients, many patients do not receive the anti-
venom they require in a timely manner [9]. The 2019 WHO strategy for a globally coordinated
response to SBE highlighted the need to prioritise clinical research into the safety and efficacy
of antivenoms [2].
An integral part of the antivenom clinical research pipeline is pre-clinical assessment
including the use of animal models. Pre-clinical assessment includes characterising the neutra-
lisation of venom induced lethality and reversal of specific toxic effects of the venom, and anti-
venomics [10, 11]. Additional quantitative clinical assessment of antivenom pharmacokinetic
properties (e.g. elimination half-life and volume of distribution) and pharmacodynamic prop-
erties (e.g. correction of coagulopathy, nephrotoxicity and haemodynamic instability) allows
for the rational design of dosing strategies [12]. This is rarely done for antivenoms. Comple-
mentary to pharmacological consideration, dose optimisation can be done via phase 2 clinical
trials. Ideally this is performed using adaptive design principles [13]. Adaptive designs are
needed because it is rarely possible to pre-specify a suitably small set of doses that satisfy rea-
sonable expectations for acceptable safety and efficacy. Many antivenoms will have narrow
therapeutic windows and cannot be ethically administered to healthy volunteers, therefore
dose optimisation trials need to simultaneously assess efficacy and toxicity.
PLOS NEGLECTED TROPICAL DISEASES
Adaptive design for antivenom dose optimisation
PLOS Neglected Tropical Diseases | https://doi.org/10.1371/journal.pntd.0008109 November 16, 2020 2 / 18
Funding: The MORU Tropical Health Network is
funded by the Wellcome Trust. TL is on a
fellowship funded by the Hamish Ogston
Foundation. The funders had no role in study
design, data collection and analysis, decision to
publish, or preparation of the manuscript.
Competing interests: The authors have declared
that no competing interests exist.

Adaptive designs for dose-finding trials are of two main types. First, rule based designs
which do not make any parametric assumptions regarding the relationship between the dose
and the outcome of interest (e.g. efficacy or toxicity). A rule based design usually only assumes
that there is a monotone increasing relationship between the dose and the outcome, i.e. the
probability of the outcome increases with higher doses. The ‘3+3’ design is the best known rule
based adaptive design [14]. The standard formulation of the ‘3+3’ design proceeds by recruit-
ing successive cohorts of 3 subjects. Dose escalation for a subsequent cohort of 3 subjects
occurs if no toxicity is observed amongst the previous 3; an additional 3 are given the same
dose if toxicity is observed in only 1 out of 3; dose de-escalation occurs if toxicity is observed
in 2 or more out of the previous 3. Rule based designs do not use information accrued across
dosing levels, and therefore they have limited ability to rapidly identify the desired optimal
dose with high confidence [15]. The alternative is a model based design, which requires deter-
mining a parametric relationship (model) between the dose and the outcome, termed a dose-
response model [16]. The continual reassessment method [17] was the first proposed model
based design for dose-finding. Data from sequentially enrolled patients are used to continually
update the parameters of the dose-response model. Each enrolled patient is then assigned the
expected optimal dose under the estimated dose-response model. The original rule based
designs [14] and model based designs [17] for dose-finding were published around the same
time. Although model based designs are more efficient, more flexible and have better operating
characteristics [18], rule-based designs have been the dominant choice. For example, fewer
than 1 in 10 trials in oncology—where dose-finding is critical—have used a model based
approach [19, 20], mostly due to perceived difficulty of implementation and lack of under-
standing of the methods [21].
This paper outlines a model based, Bayesian adaptive design for phase 2 studies with the
objective of optimising antivenom dosing. The structure of the design was motivated by the
need to determine the optimal dosing for a novel antivenom developed to treat Russell’s viper
envenoming in Myanmar. Following a recent 4-year collaborative initiative between institu-
tions in Myanmar and Australia entitled the Myanmar Snakebite project, antivenom produc-
tion facilities improved, resulting in the production of a new monospecific lyophilised F(ab)2
antivenom (Viper antivenom BPI) [22]. This new lyophilised antivenom has replaced the for-
mer liquid antivenom and is now distributed countrywide. The lyophilised antivenom has the
potential to greatly improve access to antivenom as the electrification rate in Myanmar is one
of the lowest in Asia (70% in 2017 [23]). The current dosing strategy is based on unpublished
results of pre-clinical testing and stratified into two doses according to absence or presence of
clinical features of severity at presentation (80 mL and 160 mL, respectively). No clinical trial
data or post marketing data have been published to support the efficacy or toxicity of these rec-
ommended doses.
This situation in Myanmar mirrors the development of many antivenoms worldwide [2, 24,
25] and highlights the need for high quality dose-optimisation studies. There is a need to stan-
dardise the methodology of clinical trials of antivenom whilst maintaining patient safety with
robust patient monitoring built into study design. This calls for dose-finding phase 2 trials that
can rapidly identify optimal dosing strategies, while minimising harm to patients. There are
two concurrent considerations when optimising antivenom dosing. First, the efficacy of the
dose, defined in the context of Russell’s viper envenoming as the restoration of blood coagula-
tion within 6 hours. Second, the dose-related toxicity, defined in this context as the occurrence
of an anaphylactic reaction within 180 minutes post antivenom administration. Envenoming
from different snakes will require different definitions of efficacy and toxicity. In the context
of Russell’s viper envenoming in Myanmar, the choice of these two binary clinical end-points
was pragmatic due to their clinical significance, resource availability and replication of current
PLOS NEGLECTED TROPICAL DISEASES
Adaptive design for antivenom dose optimisation
PLOS Neglected Tropical Diseases | https://doi.org/10.1371/journal.pntd.0008109 November 16, 2020 3 / 18

clinical practice. The model based design estimates dose-response curves for both the efficacy
outcome and the toxicity outcome, and thus derives a contextually defined ‘optimal dose’. The
particularities of the design reported here were tailor-made for the dose-finding trial in Daboia
siamensis envenoming but the design generalises to any systemic envenoming with clinically
relevant endpoints whereby the efficacy and toxicity outcomes are both binary, e.g. [24, 26,
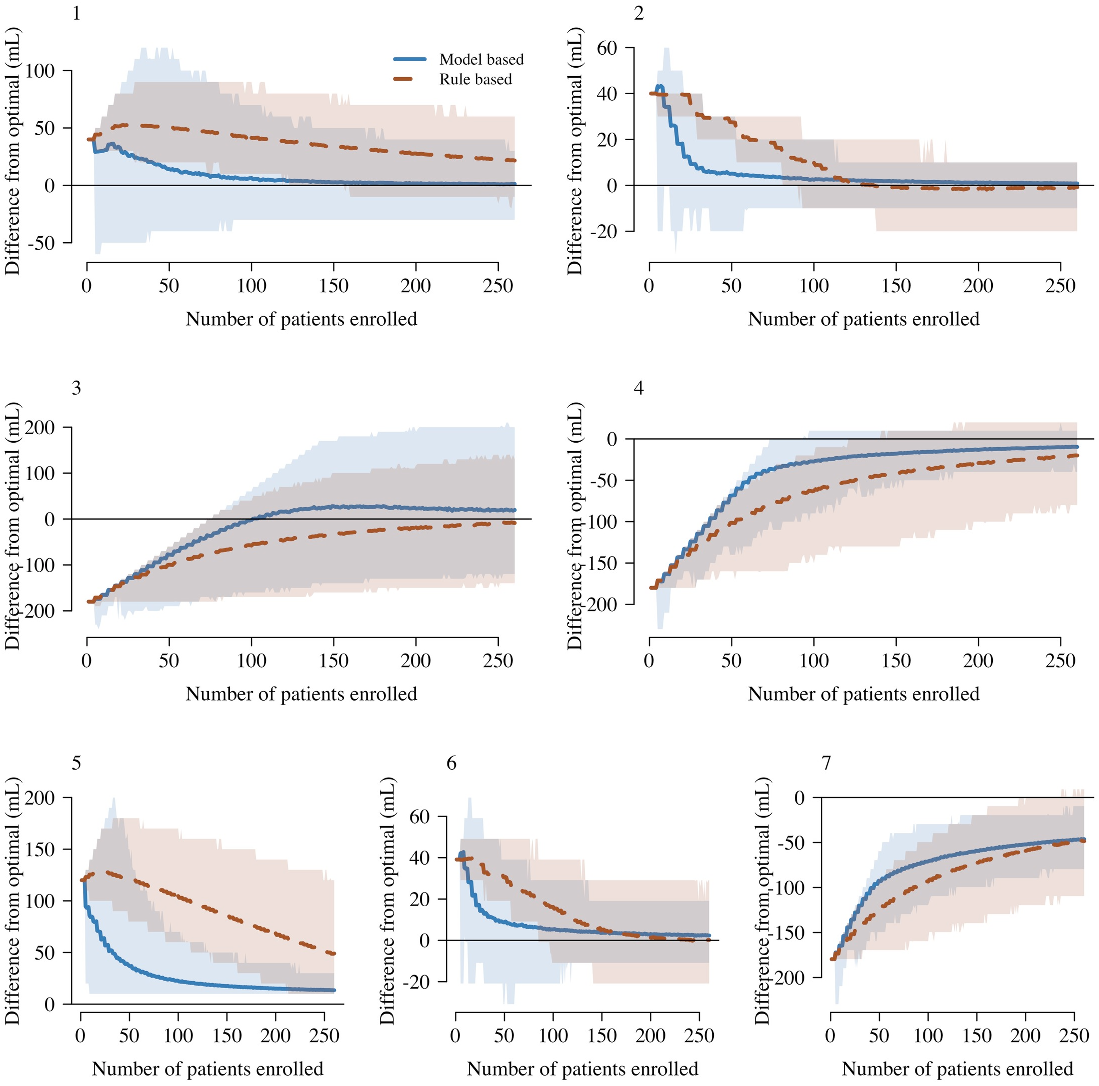
27]. We compared the in silico performance of this design against that of a tailor-made rule
based design (modified ‘3+3’ design or cumulative cohort design) under multiple simulation
scenarios. The full simulation code written in R is open access and can easily be adapted to dif-
ferent antivenoms.
Methods
Model based adaptive design for dose-finding
In this section we give an overview of how doses are adaptively chosen during the trial and
describe the necessary parameters for the adaptive assignment of doses to patients sequentially
enrolled. First it is necessary to choose a randomisation ratio between the standard of care dos-
ing arm and the adaptive dosing arm. This value can be set to 0 (i.e. all patients are assigned to
the adaptive arm). Values greater than 0 result in a fixed proportion of patients assigned to the
standard of care dose. This allows for a direct comparison (model free) between outcomes
under the standard of care dose and outcomes under the dose to which the adaptive algorithm
converges after a sufficient number of patients are enrolled. It also allows for a model free esti-
mate of the efficacy and safety of the standard of care dose. The randomisation ratio is fixed
throughout the trial.
In the adaptive arm, the adaptive dose assignment will depend on (i) the parametric dose-
response models of toxicity and efficacy; (ii) the prior distribution over the model parameters;
(iii) the toxicity and efficacy data observed for the antivenom in patients treated thus far; and
(iv) the maximum tolerated toxicity and target efficacy (see below). The dose-efficacy and
dose-toxicity models are updated using data from both the standard of care arm and the adap-
tive arm throughout the trial.
Patients are enrolled in successive cohorts of a pre-specified size N
cohort
1. The choice of
the value of N
cohort
is pragmatic as it determines how often it is necessary to update the model.
Randomisation is performed at the individual level. We assume that the toxicity and efficacy
outcomes for all previously enrolled cohorts of patients are known when a new cohort of
patients is enrolled. At the start of the trial there is a “burn-in” period (a pre-specified number
of patients). During this burn-in period, patients randomised to the adaptive arm are given the
starting dose for the adaptive arm, which is the optimal dose under the prior distribution over
the model parameters. After burn-in, patients randomised to the adaptive arm are given the
posterior predicted optimal dose under the model (the distribution over model parameters is
updated using all accrued data). If the model predicted dose is more than any previously
trialled dose plus the maximum dose increment δ
v
, then patients are given the maximum
allowed dose (the greatest previously assigned dose plus δ
v
).
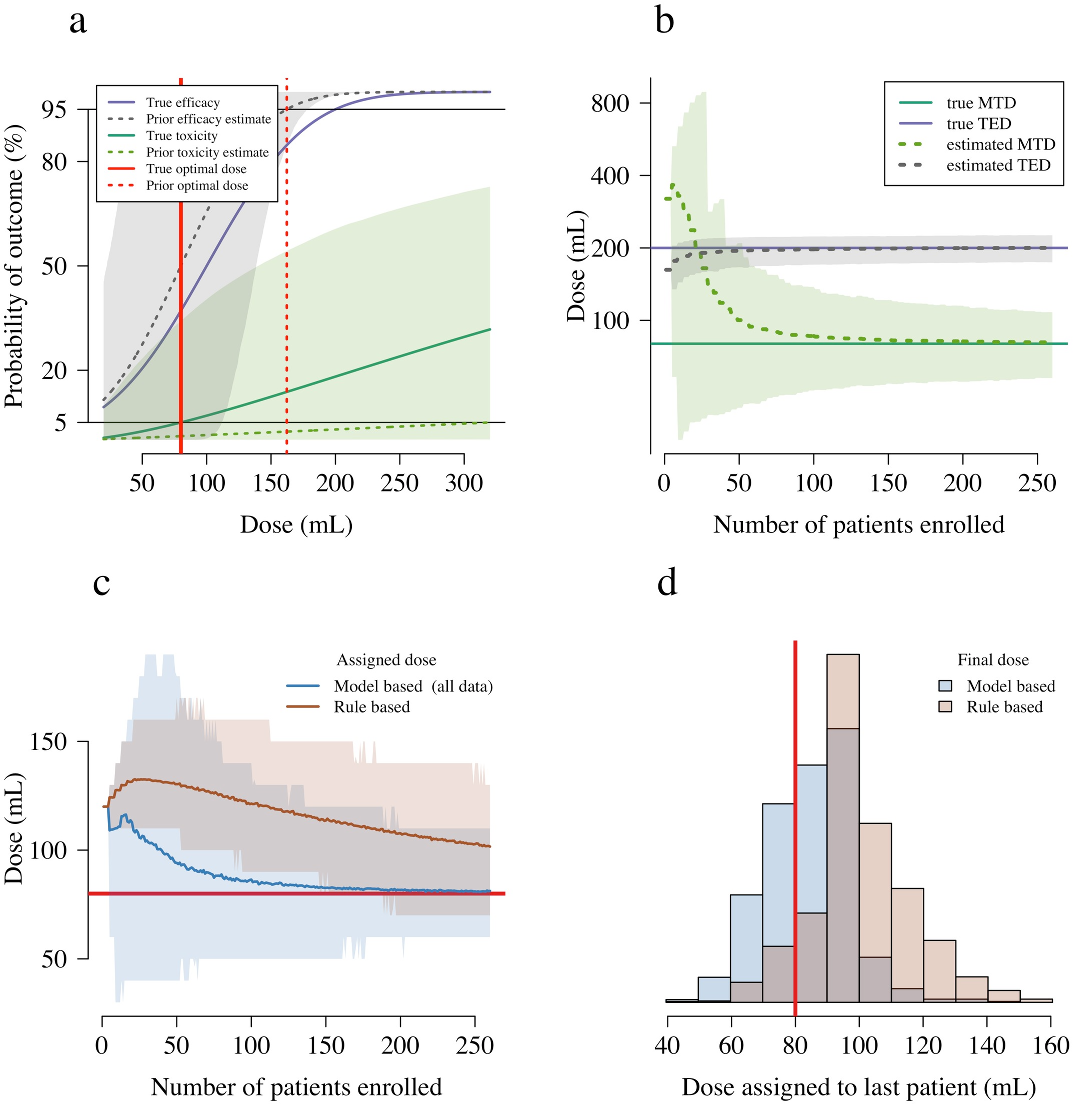
For a given distribution over the model parameters, we define the optimal dose as follows.
We first define a maximum tolerated toxicity (MTT), and a target efficacy level (TEL). The
mean posterior predicted dose that has average toxicity equal to the MTT is denoted the maxi-
mum tolerated dose (MTD); and the mean posterior predicted dose that has average efficacy
equal to the TEL is denoted the target efficacious dose (TED). The optimal dose is then defined
as: V
= min(MTD, TED).
Additional parameters in the trial could include a minimum dose (the adaptively chosen
dose cannot go below this dose); a maximum dose (the adaptively chosen dose cannot go
PLOS NEGLECTED TROPICAL DISEASES
Adaptive design for antivenom dose optimisation
PLOS Neglected Tropical Diseases | https://doi.org/10.1371/journal.pntd.0008109 November 16, 2020 4 / 18

above this dose). If a minimum or a maximum dose are defined then these should be put into
context with respect to the starting dose, the maximum dose increment or decrement and the
total sample size. The purpose of a burn-in period for the adaptive arm is to reduce stochasti-
city at the start of the trial, especially in the context of weakly informative prior distributions
over the model parameters. For example, a burn-in of 20 patients would imply that the adap-
tive arm would only be updated after the first 20 patients had been enrolled (irrespective of
how they were randomised).
In addition, it is possible to specify stopping rules for the trial. For example, randomisation
to the control arm (standard of care dose) could be stopped once sufficient evidence of its infe-
riority has been accrued (either too low and thus inferior efficacy, or too high and thus inferior
due to excess toxicity) in comparison to the current adaptive dose. We would recommend the
use of a non-parametric test (e.g. Fisher’s exact test), with appropriate adjustment for multiple
testing.
It may be the case that the antivenom used in the trial is from multiple batches. Batch varia-
tion can be an important contributor to variability in both toxicity and efficacy. It is straight-
forward to add a batch variation term in the adaptive models of efficacy and toxicity. This is an
advantage of a model based design over a rule based design.
Parametric models of toxicity and efficacy. The model based adaptive design necessi-
tates determining parametric dose-response relationships for both the dose-related toxicity
and the dose-related efficacy. We model both the efficacy and toxicity outcomes as Bernoulli
random variables with dose-dependent parameters estimated under a generalised linear
model. For the efficacy outcome we use the probit link function, and for the toxicity outcome
we use the logistic link function.
The use of the probit link for the efficacy dose-response is motivated by a mechanistic
understanding of how the antivenom acts. Assuming (i) there is a fixed linear relationship
between the volume of venom in the body (which is unknown) and the dose of antivenom
needed to neutralise all the circulating venom, and (ii) that the total mass of venom injected is
approximately normally distributed, then the efficacy dose-response curve follows a normal
cumulative distribution with mean μ and standard deviation σ (i.e. probit link with parameters
μ, σ). The parameter μ corresponds to an efficacious dose of antivenom in 50% of patients. The
value of μ + 1.64σ corresponds to an efficacious dose of antivenom in approximately 95% of
patients. Weakly informative and interpretable priors can be set for both these parameters.
We choose to model the dose-toxicity relationship using a logistic function, where the dose
is modelled on the logarithmic scale (base 2 for visual purposes, this does not impact the statis-
tical inference). This is equivalent to fitting a Bayesian logistic regression model to the toxicity
outcomes. Additional details of the Bayesian adaptive design are given in S1 Text.
A modified ‘3+3’ rule based design
In order to illustrate the advantages and disadvantages of model based adaptive designs, we
compared the in silico performance of our model based adaptive design with that of a modified
‘3+3’ rule based design. As in our model based design, patients are recruited in cohorts of size
N
cohort
. This is set to 3 in the classic ‘3+3’ design, but in our case is a trial design parameter.
The rule based design does not make parametric assumptions about the relationship between
the dose and the outcomes. For each dose v trialled, a dose-dependent frequentist estimate of
toxicity,
^
y
tox
v
, and a dose-dependent frequentist estimate of efficacy,
^
y
eff
v
, are calculated. Based
on these estimates, the dose is subsequently increased, decreased, or remains the same for the
next N
cohort
patients, according to a pre-specified set of rules and trial design parameters (the
MTT and the TEL). Our rule based design is a type of cumulative cohort design [28] as it uses
PLOS NEGLECTED TROPICAL DISEASES
Adaptive design for antivenom dose optimisation
PLOS Neglected Tropical Diseases | https://doi.org/10.1371/journal.pntd.0008109 November 16, 2020 5 / 18