Abstract: La contaminacion de suelos por metales pesados se esta convirtiendo en una preocupacion ambiental en las ultimas decadas debido al incremento de las actividades industriales y al progreso de la agricultura, que pueden provocar que se eleven sus concentraciones hasta niveles potencialmente fitotoxicos, provocando una perdida de la calidad del suelo y limitando su uso. Esto tambien puede afectar a la calidad de las aguas, tanto superficiales como freaticas, ampliando asi el impacto sobre el medio natural.
Los metales pesados no son biodegradables, y por tanto, su presencia en suelos, rios y lagos puede facilitar su acumulacion en los organismos y, de este modo, llegar a la cadena trofica. Existen diferentes tecnicas tradicionales para la eliminacion de metales pesados en aguas y suelos, entre las que se encuentran el intercambio ionico, la filtracion por membrana, la electrolisis, la coagulacion, la flotacion y la adsorcion. Sin embargo, tienen varias desventajas como su alto coste de operacion y la generacion de lodos. Entre estos metodos, la adsorcion es el mas empleado para la retencion de metales pesados y otros contaminantes.
La bioadsorcion es una mecanismo ecologico, sostenible, rapido y economico para tratar de inmovilizar contaminantes. Diferentes tipos de biomasa vegetal se utilizan frecuentemente como bioadsorbentes en suelos y aguas residuales, puesto que son facilmente accesibles en la naturaleza y de bajo coste, y habitualmente se trata de residuos o subproductos de la industria agro-alimentaria y forestal. Asi, su aplicacion al suelo para la inmovilizacion de metales pesados presentaria dos ventajas: en primer lugar, el posible aumento de la capacidad de adsorcion de estos metales por los suelos y la consiguiente reduccion de su presencia en las aguas de drenaje y, en segundo lugar, se le proporcionaria un valor anadido a estos residuos.
En la actualidad, la produccion global de madera alcanza anualmente mas de 2.000 millones de m3, de los cuales entre el 10 y el 22% es corteza. Espana es el cuarto pais europeo con mayor superficie forestal (despues de Suecia, Finlandia y Francia), con una extension forestal del 29% de la superficie total, y con mas de 5,2 millones de hectareas de bosques de coniferas, principalmente pinos (Pinus pinaster, P. radiata, P. pinea, P. sylvestris). La corteza de pino esta constituida basicamente por lignina, celulosa y taninos. Se ha demostrado que los residuos agricolas con un alto contenido en celulosa son eficaces en la retencion de metales en disoluciones acuosas, por lo que la corteza de pino podria ser una alternativa a las tecnicas tradicionales para la inmovilizacion de metales pesados en aguas y suelos, dada su condicion de bioadsorbente de bajo coste. Esto significaria una manera efectiva de recuperar y reutilizar un residuo de escaso valor economico para solventar un problema ambiental.
Los suelos pueden presentar una elevada concentracion de metales debido a causas naturales, pero son las causas antropicas como la mineria, la contaminacion de industrias proximas, la adicion de estiercoles y purines (en suelos de praderia) o la aplicacion de fungicidas (como es el caso de los suelos dedicados al cultivo de la vid) las que realmente incrementan de forma significativa los niveles de metales pesados en los suelos por encima de los valores de fondo.
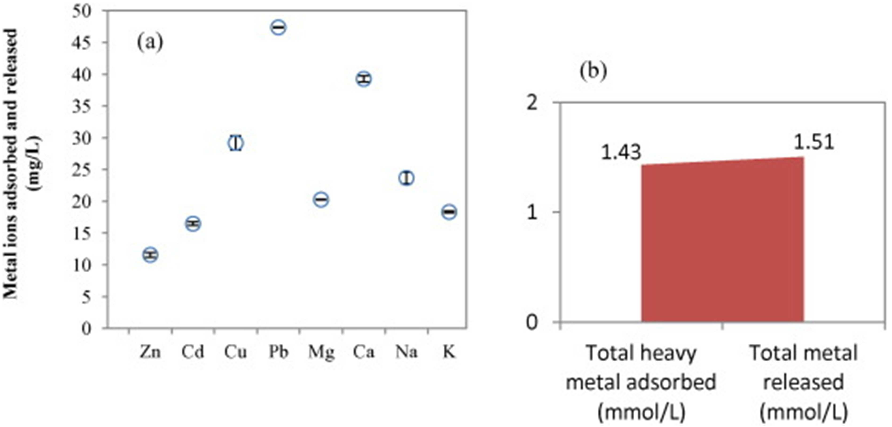
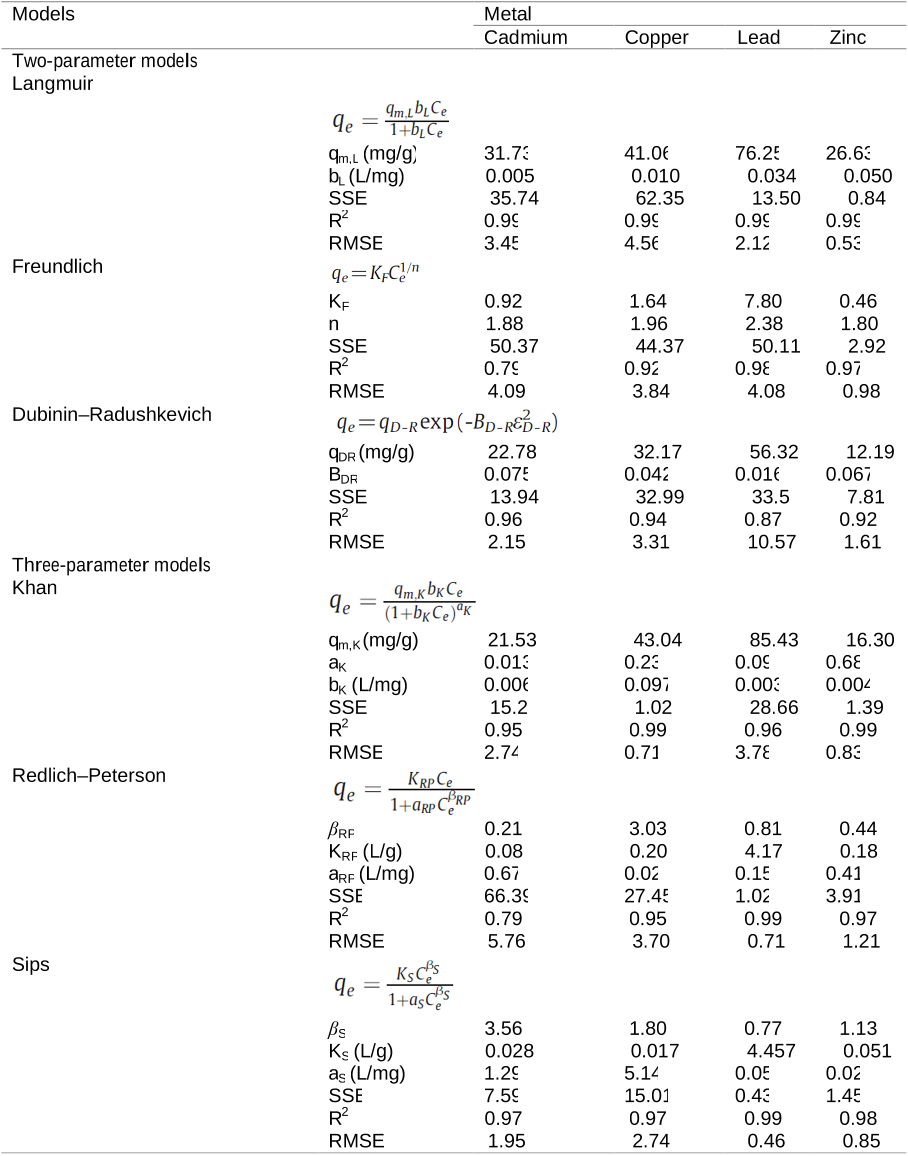
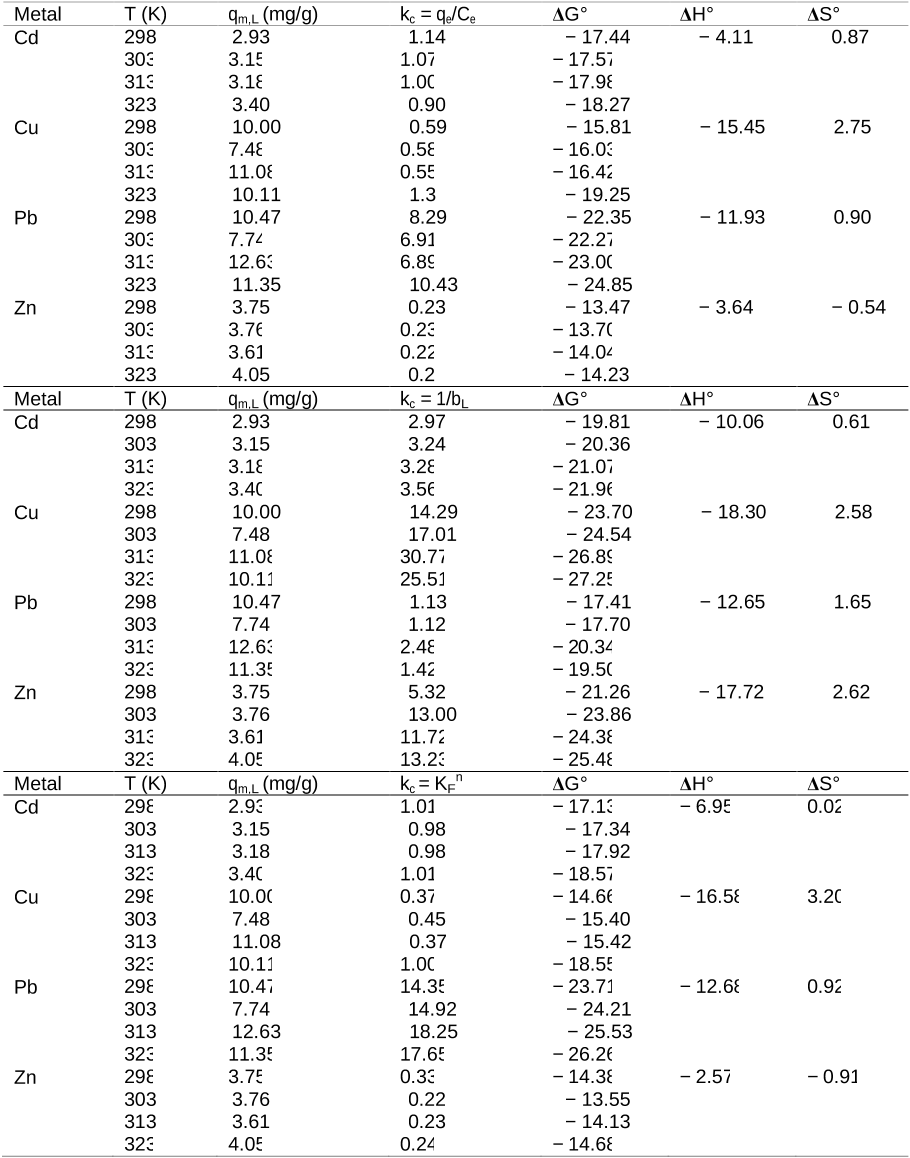
En esta Tesis se evaluo la capacidad de la corteza de pino para adsorber cinco metales pesados (Cd, Cu, Ni, Pb y Zn) mediante diversos experimentos (discontinuos tipo batch, continuos en reactor de flujo agitado y en columnas, y en presencia de uno o varios metales simultaneamente). A continuacion, se estudiaron los efectos de la aplicacion de este adsorbente natural en dos suelos con un alto contenido en cobre. Asi, mediante experimentos en macetas, se evaluaron los efectos de la adicion de corteza de pino a un suelo de vinedo sobre el desarrollo de una especie forrajera, el Lolium perene. Adicionalmente, tambien se comprobaron los efectos sobre el crecimiento de esta planta como consecuencia de la adicion combinada de corteza de pino y concha de mejillon. Posteriormente, en un suelo de escombrera de mina de cobre, se estudio el efecto individualizado de la adicion de corteza de pino, y de esta combinada con concha de mejillon, sobre el fraccionamiento y la desorcion de los cinco metales antes citados junto con su influencia en el crecimiento bacteriano y fungico. En un estudio complementario, se llevo a cabo el diagnostico de los niveles de contaminacion de metales pesados en suelos forestales (bosques caducifolios) y suelos de pradera en las cercanias de una planta cementera, utilizando como referencia los niveles fondo que esos metales presentaban en la litologia de la zona. Este procedimiento de diagnosis de la contaminacion deberia considerarse como una etapa inicial, para posteriormente evaluar la utilidad del aporte de la corteza de pino en la inmovilizacion de metales pesados en condiciones de campo.
Los materiales de desecho de las minas abandonadas, especialmente en aquellas donde se explotaron diferentes metales pesados, facilitan la contaminacion de los suelos adyacentes, debido a la deposicion de particulas cargadas de metales pesados que son transportadas por el viento y a la presencia de elevadas concentraciones de metales en aguas superficiales y freaticas. Todo esto, ademas, incrementa el riesgo ambiental al que estan expuestas las areas urbanas y agricolas del entorno de estas antiguas explotaciones. La contaminacion por metales pesados es uno de los principales impactos medioambientales, especialmente porque facilita su presencia en forma soluble tanto en las aguas superficiales como subterraneas, pero tambien porque en estos medios puede ocurrir el transporte de coloides ricos en metales pesados.
Por otro lado, en los suelos dedicados al vinedo, se encontraron concentraciones de Cu muy elevadas (en muchas ocasiones superando los 200 mg kg-1), consecuencia directa del continuo e intenso tratamiento de las enfermedades fungicas de la vid con fitosanitarios de base cuprica. Las condiciones climaticas en Galicia (temperaturas suaves y elevada humedad), y la situacion de gran parte de los vinedos cerca de los principales rios (Mino-Sil), provocan que las vides esten expuestas en mayor medida al desarrollo de enfermedades fungicas. En estos suelos dedicados al cultivo de la vid, la contaminacion por cobre supone una seria amenaza a su calidad debido a la toxicidad y persistencia en el medio edafico, pudiendo ocasionar efectos graves sobre las plantas y los microorganismos del suelo.
Ademas de los suelos de vinedo, suelos dedicados a otras actividades del sector primario como los suelos de praderia o los suelos forestales, tambien pueden ser susceptibles de recibir aportes de metales pesados originados en instalaciones industriales proximas. Un ejemplo es el caso de los suelos del entorno de las plantas cementeras. Por otro lado, la posible existencia de elevados niveles de metales pesados en suelos de praderas puede obedecer a su presencia como impurezas en enmiendas (estiercol, purines, etc.) que se anaden al suelo con la finalidad de satisfacer las necesidades nutricionales de la cobertura vegetal. No obstante, tasas de adicion excesivas o inadecuadas de estas enmiendas podrian contribuir a aumentar los niveles de metales, originando la contaminacion del suelo y facilitando su posible transferencia hacia aguas superficiales y subterraneas.
Las cementeras emiten numerosos contaminantes a la atmosfera durante el proceso de elaboracion del cemento, entre los que se encuentran algunos metales pesados. Esto se debe fundamentalmente a la quema de los combustibles fosiles empleados para el calentamiento de los hornos y a la liberacion de los metales presentes en las materias primas. En ocasiones, estas instalaciones tambien emplean combustibles alternativos (biogas, y residuos como neumaticos, plasticos, etc.) como sustitutos o complementos de los tradicionales, lo que tambien puede contribuir a la emision de otro tipo de contaminantes (PAHs, PCBs, dioxinas, etc). El destino de los metales pesados movilizados durante la elaboracion del cemento es incorporarse al producto final, ser retenidos en los sistemas de reduccion de las emisiones o ser emitidos a la atmosfera. En este ultimo caso, los metales pesados se depositan finalmente a diferentes distancias de la fuente de emision, donde se acumulan en suelos y vegetacion.
Asi, en un marco europeo en el que cada vez se reclama con mayor insistencia una actividad agricola sostenible, esta Tesis tratara de evaluar la problematica de la contaminacion por metales pesados en suelos con diferente uso, y la posible utilizacion de un subproducto como la corteza de pino como un bioadsorbente de metales pesados efectivo y de bajo coste.