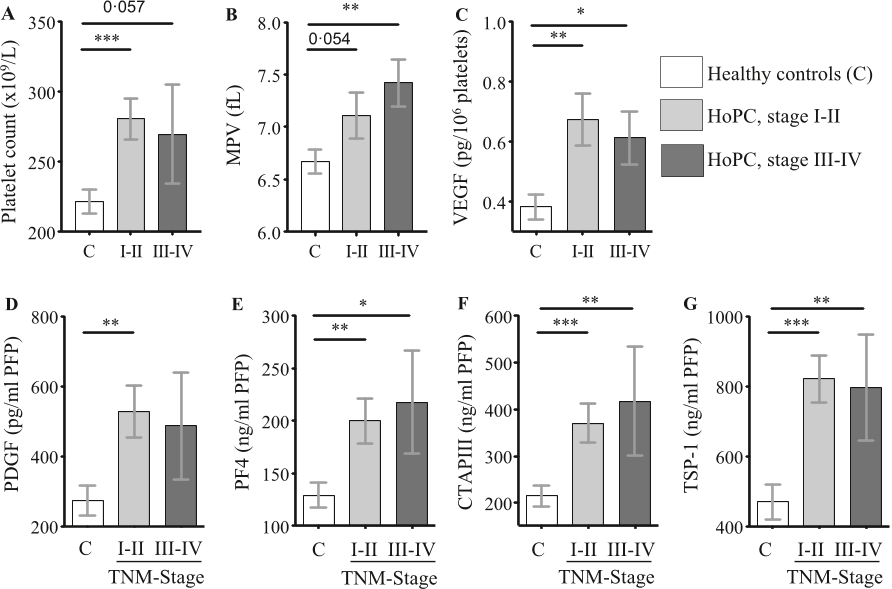
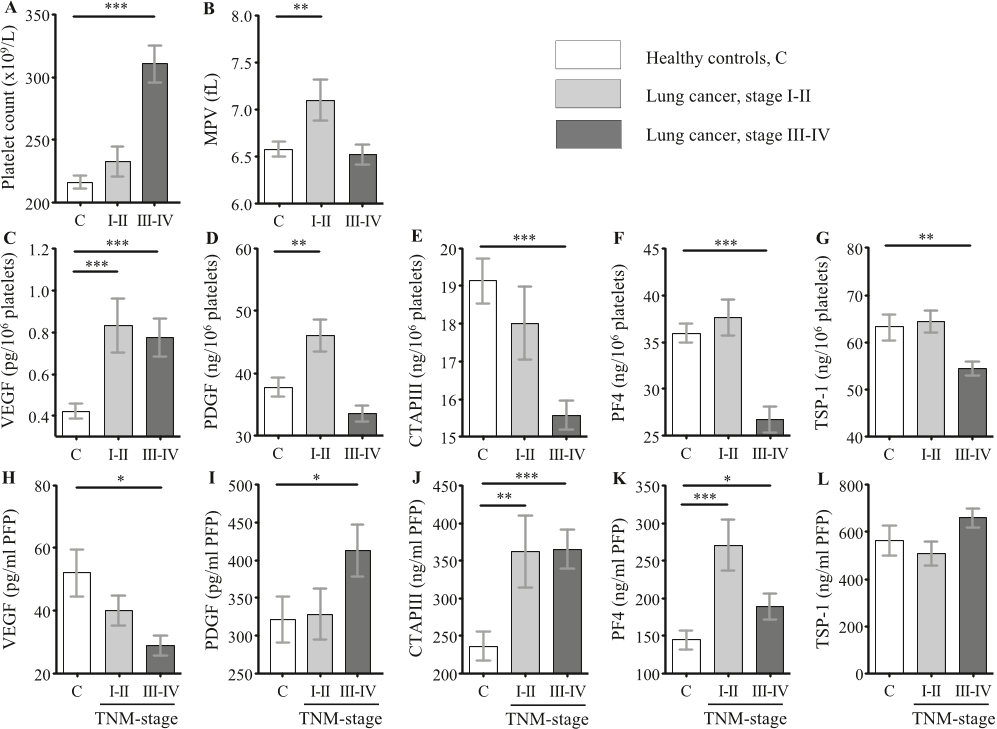
Q2. What future works have the authors mentioned in the paper "A combination of platelet features allows detection of early-stage cancer" ?
Future research is needed to further investigate the clinical relevance of their findings. Platelets are a new and uncharted source of information, which need to be further explored in blood-based biomarker research.