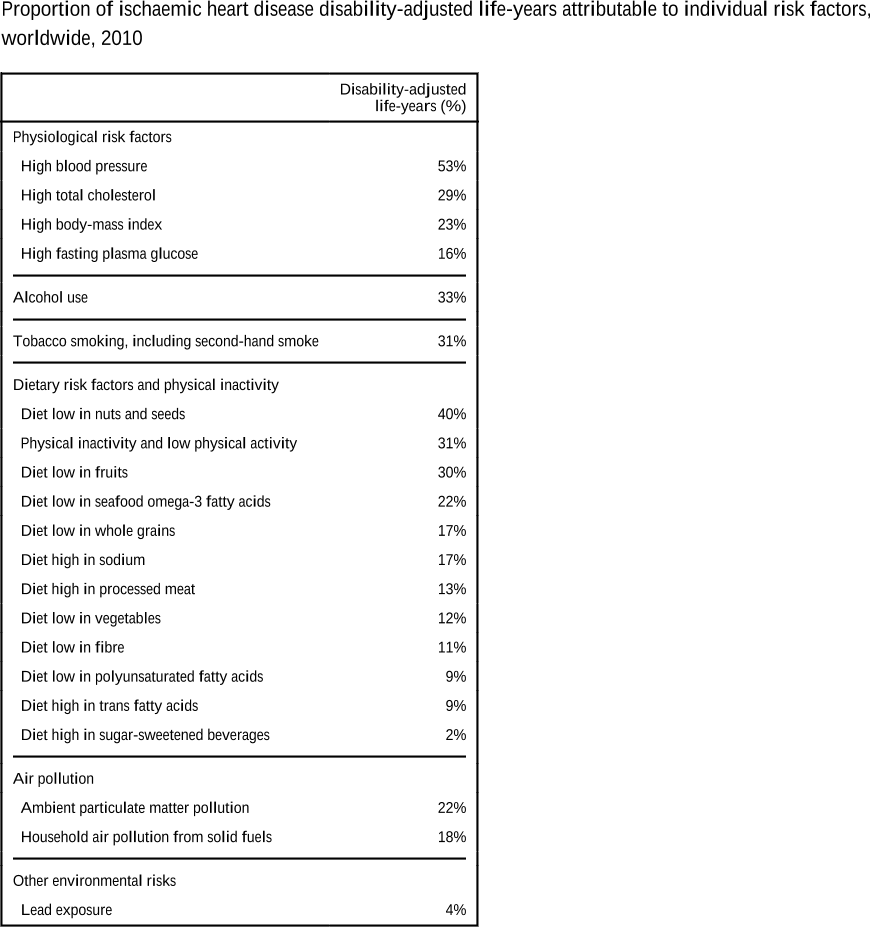
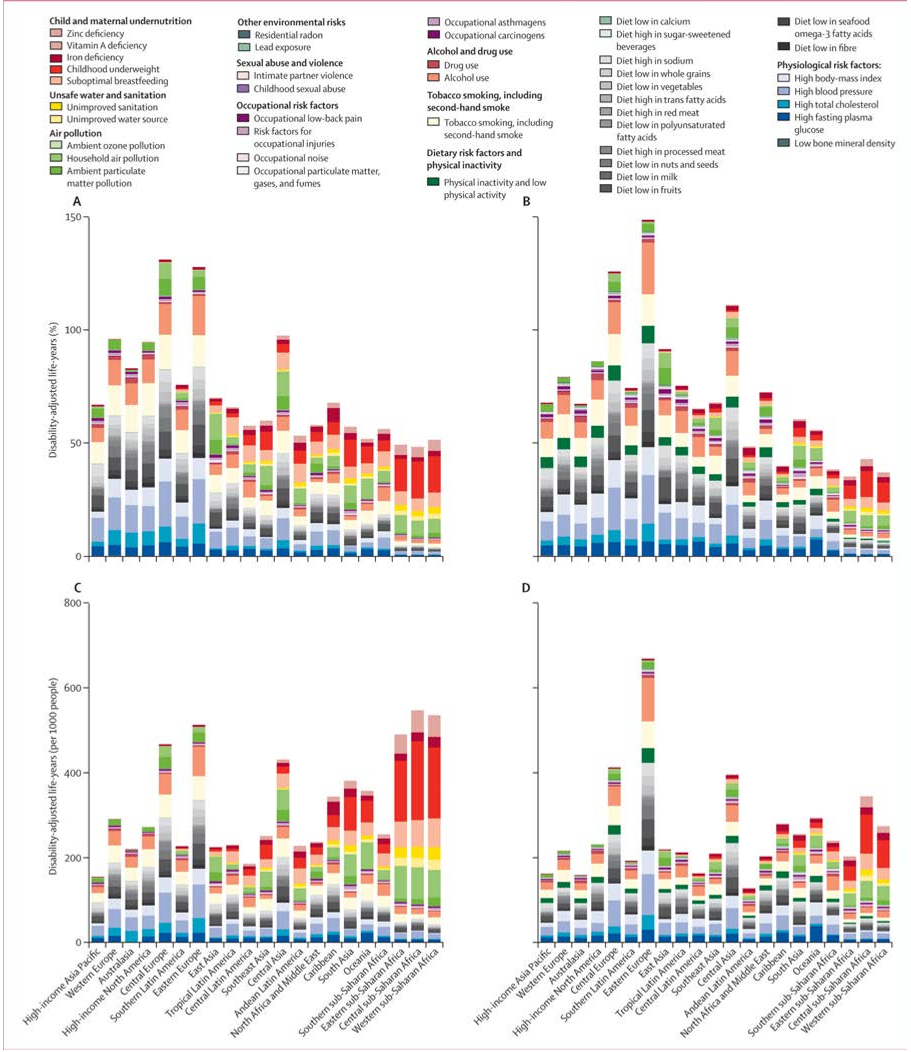
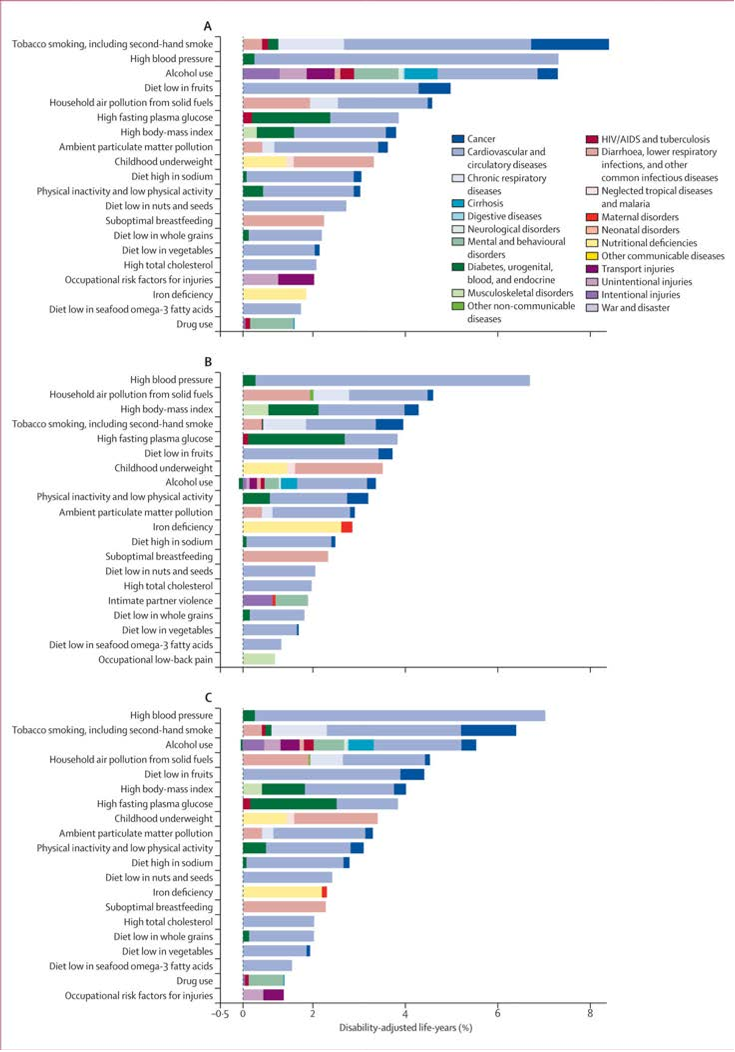
Abstract: Importance In the United States, national associations of individual dietary factors with specific cardiometabolic diseases are not well established. Objective To estimate associations of intake of 10 specific dietary factors with mortality due to heart disease, stroke, and type 2 diabetes (cardiometabolic mortality) among US adults. Design, Setting, and Participants A comparative risk assessment model incorporated data and corresponding uncertainty on population demographics and dietary habits from National Health and Nutrition Examination Surveys (1999-2002: n = 8104; 2009-2012: n = 8516); estimated associations of diet and disease from meta-analyses of prospective studies and clinical trials with validity analyses to assess potential bias; and estimated disease-specific national mortality from the National Center for Health Statistics. Exposures Consumption of 10 foods/nutrients associated with cardiometabolic diseases: fruits, vegetables, nuts/seeds, whole grains, unprocessed red meats, processed meats, sugar-sweetened beverages (SSBs), polyunsaturated fats, seafood omega-3 fats, and sodium. Main Outcomes and Measures Estimated absolute and percentage mortality due to heart disease, stroke, and type 2 diabetes in 2012. Disease-specific and demographic-specific (age, sex, race, and education) mortality and trends between 2002 and 2012 were also evaluated. Results In 2012, 702 308 cardiometabolic deaths occurred in US adults, including 506 100 from heart disease (371 266 coronary heart disease, 35 019 hypertensive heart disease, and 99 815 other cardiovascular disease), 128 294 from stroke (16 125 ischemic, 32 591 hemorrhagic, and 79 578 other), and 67 914 from type 2 diabetes. Of these, an estimated 318 656 (95% uncertainty interval [UI], 306 064-329 755; 45.4%) cardiometabolic deaths per year were associated with suboptimal intakes—48.6% (95% UI, 46.2%-50.9%) of cardiometabolic deaths in men and 41.8% (95% UI, 39.3%-44.2%) in women; 64.2% (95% UI, 60.6%-67.9%) at younger ages (25-34 years) and 35.7% (95% UI, 33.1%-38.1%) at older ages (≥75 years); 53.1% (95% UI, 51.6%-54.8%) among blacks, 50.0% (95% UI, 48.2%-51.8%) among Hispanics, and 42.8% (95% UI, 40.9%-44.5%) among whites; and 46.8% (95% UI, 44.9%-48.7%) among lower-, 45.7% (95% UI, 44.2%-47.4%) among medium-, and 39.1% (95% UI, 37.2%-41.2%) among higher-educated individuals. The largest numbers of estimated diet-related cardiometabolic deaths were related to high sodium (66 508 deaths in 2012; 9.5% of all cardiometabolic deaths), low nuts/seeds (59 374; 8.5%), high processed meats (57 766; 8.2%), low seafood omega-3 fats (54 626; 7.8%), low vegetables (53 410; 7.6%), low fruits (52 547; 7.5%), and high SSBs (51 694; 7.4%). Between 2002 and 2012, population-adjusted US cardiometabolic deaths per year decreased by 26.5%. The greatest decline was associated with insufficient polyunsaturated fats (−20.8% relative change [95% UI, −18.5% to −22.8%]), nuts/seeds (−18.0% [95% UI, −14.6% to −21.0%]), and excess SSBs (−14.5% [95% UI, −12.0% to −16.9%]). The greatest increase was associated with unprocessed red meats (+14.4% [95% UI, 9.1%-19.5%]). Conclusions and Relevance Dietary factors were estimated to be associated with a substantial proportion of deaths from heart disease, stroke, and type 2 diabetes. These results should help identify priorities, guide public health planning, and inform strategies to alter dietary habits and improve health.