




Did you find this useful? Give us your feedback









42 citations
12 citations
6 citations
5 citations
3 citations
12 citations
...In recent years, many radiochemical procedures based on Ni resin have been applied on many nuclear materials [5, 6, 12, 13, 15, 17, 27]....
[...]
...It corresponds to the radiochemical method described by Eichrom Technologies [26] and applied in many reported works [6, 12, 13, 15, 17, 27]....
[...]
...Some authors also prepared in-house Ni resins which relies on the same principle [15, 27]....
[...]
9 citations
...Aliquots were prepared for the determination of stable metal elements and gamma emitting radionuclides by ICP-AES and gamma spectrometers respectively....
[...]
...In recent years, many radiochemical procedures based on Ni resin have been applied on many nuclear materials [5, 6, 12, 13, 15, 17, 27]....
[...]
...This hypothesis was confirmed by gamma and ICP-AES measurements....
[...]
...The recovery yield of the overall radiochemical procedure is generally determined from the measurement of stable Ni by atomic absorption spectroscopy (AAS) [12] or inductively coupled plasma-atomic emission spectroscopy (ICP-AES) [5, 13, 15, 17]....
[...]
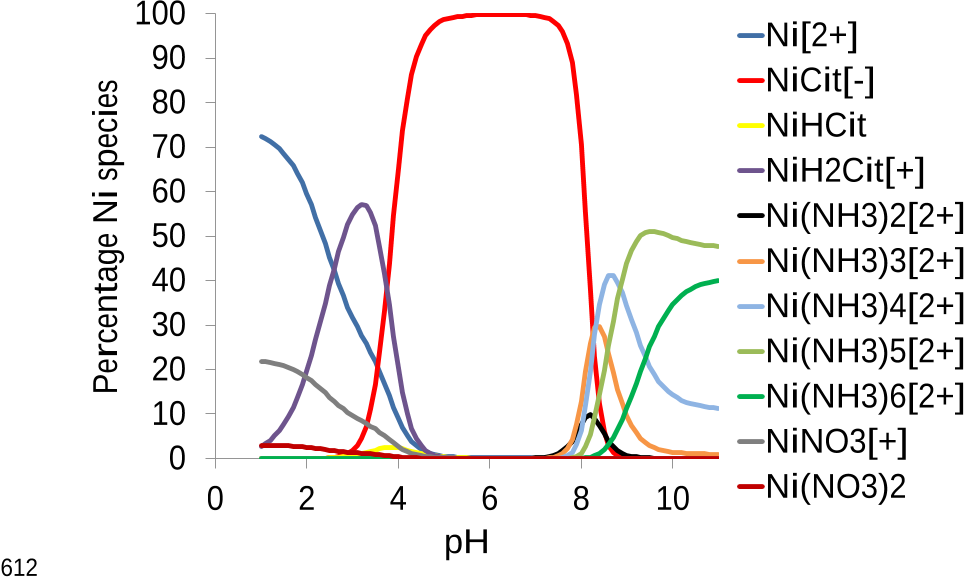
...Organic complexing agents, such as citric acid [6, 12, 21], tartaric acid [9, 21] or oxalic acid [5] are generally introduced to prevent the precipitation of Fe and the other metal elements at basic pH....
[...]
8 citations
8 citations
...Ni is then back-extracted in aqueous solution, mostly with hydrochloric acid [11, 16, 18]....
[...]
...The Ni(DMG)2 complex is first extracted in an organic solvent [20], commonly chloroform [8, 10, 11, 18, 20] which has a higher Ni extraction capacity [20]....
[...]
7 citations
...Aliquots were prepared for the determination of stable metal elements and gamma emitting radionuclides by ICP-AES and gamma spectrometers respectively....
[...]
...In recent years, many radiochemical procedures based on Ni resin have been applied on many nuclear materials [5, 6, 12, 13, 15, 17, 27]....
[...]
...This hypothesis was confirmed by gamma and ICP-AES measurements....
[...]
...The recovery yield of the overall radiochemical procedure is generally determined from the measurement of stable Ni by atomic absorption spectroscopy (AAS) [12] or inductively coupled plasma-atomic emission spectroscopy (ICP-AES) [5, 13, 15, 17]....
[...]
...Stable Fe, Co and Ni concentrations were measured using an ICP-AES (Inductively Coupled Plasma-Atomic Emission Spectroscopy) Activa M spectrometer (HORIBA Jobin– Yvon, Longjumeau, France)....
[...]