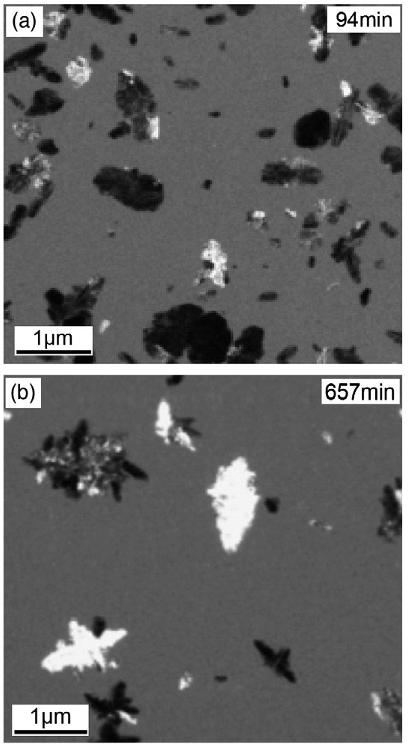
Abstract: Utilizing the concepts of a critical crystallite size and local film inhomogeneity, it is shown that nucleation in thermally annealed hydrogenated amorphous silicon occurs in the more well ordered spatial regions in the network, which are defined by the initial inhomogeneous H distributions in the as-grown films. Although the film H evolves very early during annealing, the local film order is largely retained in the still amorphous films even after the vast majority of the H is evolved, and the more well ordered regions which are the nucleation center sites for crystallization are those spatial regions which do not initially contain clustered H, as probed by H NMR spectroscopy. The sizes of these better ordered regions relative to a critical crystallite size determine the film incubation times (the time before the onset of crystallization). Changes in film short range order upon H evolution, and the presence of microvoid type structures in the as grown films play no role in the crystallization process. While the creation of dangling bonds upon H evolution may play a role in the actual phase transformation itself, the film defect densities measured just prior to the onset of crystallization exhibit no trends which can be correlated with the film incubation times.