




Did you find this useful? Give us your feedback










32 citations
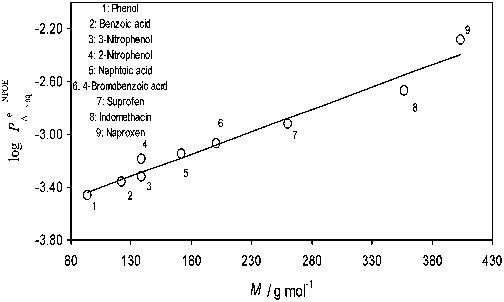
...naproxen, pirprofen, flurbiprofen, ibuprofen, carprofen, indomethacin, phenylbutazone, sulfinpyrazone, warfarin, phenobarbital, phenytoin, maleate [215], SbCl6 − and AuCl4 − [384]....
[...]
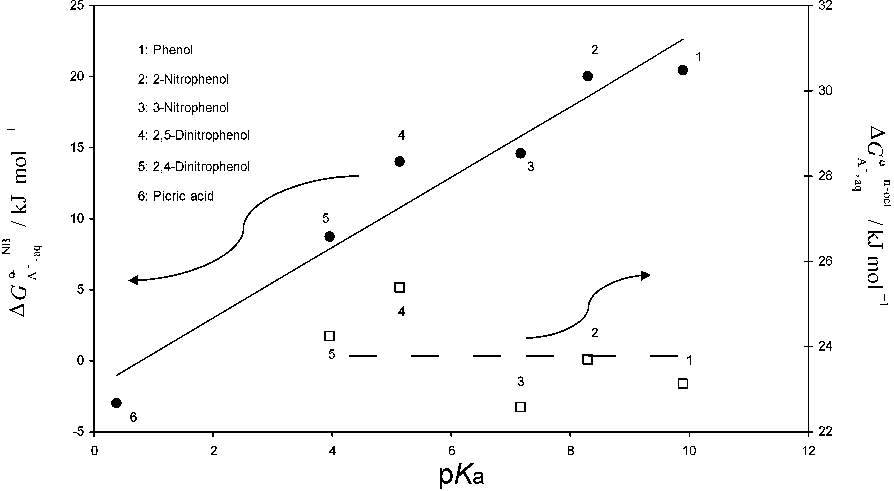
...The transfer across the water|nitrobenzene interface has been studied in the following anions: octoate [201] dodecylsulfate [201, 202]; picrate [98, 201–215]; ClO4 − [98, 147, 201, 205, 207–211, 214, 216–234]; I− [98, 147, 213, 217–219, 222, 226, 233, 235]; Br− [98, 147, 207, 213, 214, 218, 225–227, 232, 233, 235]; SCN− [98, 207, 213, 214, 217, 218, 224–228, 232, 233, 235]; NO3 − [98, 147, 207, 214, 216–219, 223, 225–228, 232–235]; IO4 − [147, 217, 218, 236]; BF4− [147, 217, 218, 222, 224]; ClO3 − [147, 218, 236]; BrO3− [147, 236]; SO42− [231, 237]; TClPB−, TFPB− [237]; carboxilate and sulphonate anions [238]; hetero- and isopolyanions [239–248]; MnO4 − [209]; Fe(CN)6 3− [223]; PF6− [224, 232]; Cl− [214, 225–228, 233, 249, 250]; acetate [214, 225, 233, 236]; hydrogenmalonate, hydrogenmaleate, hydrogensuccinate, hydrogencitraconate, hydrogenglutarate, phenolate, 2-nitrophenolate, 2methylphenolate, benzoate, salicylate, acetylsalicilate, 2clorophenolate [228]; formiate, propionate, butyrate, valeriate, capronate, oenanthate, caprylate, pelargonate, caprinate [228, 236, 251], IO3 −, OCN−, SeCN−, CN−, N3−, monofluoroacetate, difluoroacetate, trifluoroacetate, monochloroacetate, dichloroacetate, monobromoacetate, dibromoacetate, tribromoacetate, monoiodoacetate, cyclopropane carboxylate, cyclobutane carboxylate, cyclopentane carboxylate, cyclohexane carboxylate, cycloheptane carboxylate [236]; TPB−, dipicrylaminate [213]; amino acid and peptide anions [252, 253]; 3-nitrophenolate, 4-nitrophenolate, 2,4-dinitrophenolate, 2,5-dinitrophenolate, naphtoate, 4bromobenzoate, 4-chlorobenzoate, 3-chlorobenzoate, 4iodobenzoate, ketoprofen, suprofen, naproxen, pirprofen, flurbiprofen, ibuprofen, carprofen, indomethacin, phenylbutazone, sulfinpyrazone, warfarin, phenobarbital, phenytoin [215]; CF3SO3 − [231]; 4-octylbenzenesulfonate, p-toluenesulfonate [254]; F− and H2PO4− [250]....
[...]
...The transfer across the water|1,2-dichloroethane interface has been studied in the anions that follow: I− [217, 222, 226, 227, 293– 295]; ClO4 − [210, 217, 222, 226, 227, 294–297]; NO3− [217, 222, 227, 294] SCN− [217, 226, 227, 294]; IO4− [217]; BF4 − [217, 222]; picrate [210, 294, 298–300]; dodecylsulfate [301]; sulphonate anions (RSO3 −) [222]; TPB− [91, 294, 295, 297, 299, 302]; Cl− [226, 227, 249, 293, 295]; Br− [226, 227, 293–295]; rose bengal [303–305]; hetero- and isopolyanions [306]; trifluoroacetylacetone [307]; eosin B [305, 308, 309]; methyl-orange, ethyl-orange [310]; phenolate, 2-nitrophenolate, 3-nitrophenolate, 4-nitrophenolate, 2,5-dinitrophenolate [311]; lauric acid, diclofenac [312]; 2,4dinitrophenolate [294, 300, 311]; anionic drugs [215, 313]; 1-pyrene sulfonate anion [314]; bromophenol blue [315]; erythosine B, eosin Y [316]; 4-octylbenzenesulfonate, p-toluenesulfonate [254]; sulforhodamine 101 [317]; PF6 − [297]; tetrakis(pentafluorophenyl)borate, HO− [295], AuCl4 − and AuBr4− [318]....
[...]
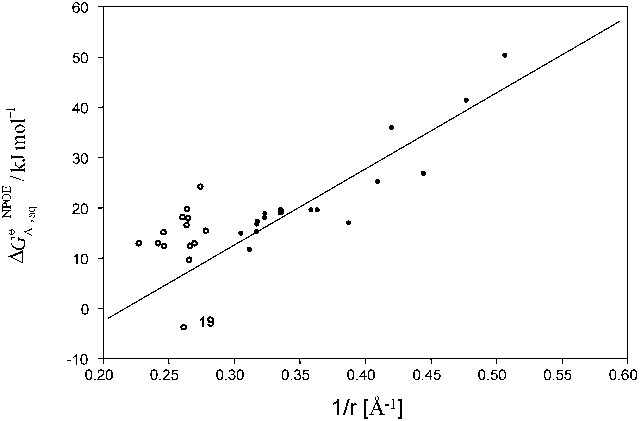
...The transfer across the water|o-nitrophenyloctylether interface has been studied in the following anions: ClO4 − [378–382]; picrate [215, 378, 380, 381, 383, 384]; TPB− [322, 379, 382, 383]; Cl− [380–382]; Br− [380, 382]; I− [380, 381], NO3−, SCN− [380–382]; sulphonate anions (R-SO3 −) [380]; 2,4-dinitrophenolate [215, 385, 386], phenolate, 2-nitrophenolate, 3-nitrophenolate, 4-nitrophenolate, 2,5-dinitrophenolate, benzoate, naphtoate, 4-bromobenzoate, 4-chlorobenzoate, 3-chlorobenzoate, 4-iodobenzoate, ketoprofen, suprofen,...
[...]
32 citations
30 citations
28 citations
28 citations
4,247 citations
1,904 citations
672 citations
555 citations
271 citations