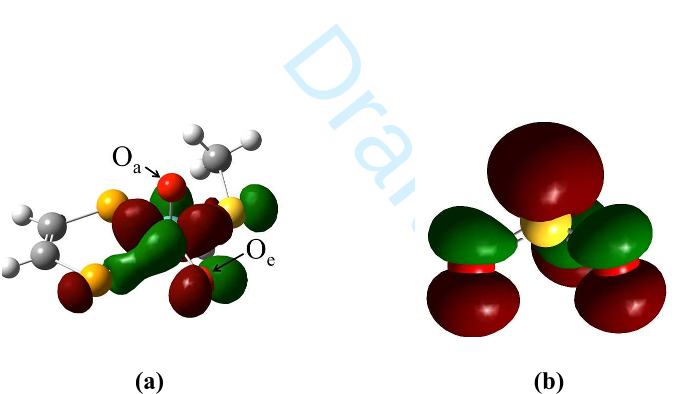
Draft
A Computational Investigation Into The Catalytic Activity Of
A Diselenolene Sulfite Oxidase Biomimetic Complex
Journal:
Canadian Journal of Chemistry
Manuscript ID
cjc-2016-0244.R1
Manuscript Type:
Article
Date Submitted by the Author:
21-Jul-2016
Complete List of Authors:
Bushnell, Eric; Brandon University, Chemistry
Keyword:
diselenolene, density functional theory, computational, dithiolene, sulfite
oxidase biomimetic complex
https://mc06.manuscriptcentral.com/cjc-pubs
Canadian Journal of Chemistry

Draft
1
A Computational Investigation Into The Catalytic Activity Of A Diselenolene
Sulfite Oxidase Biomimetic Complex
Eric A. C. Bushnell*
Department of Chemistry, Brandon University, 270-18th Street, Brandon, Manitoba R7A 6A9,
Canada.
Author to whom correspondence should be addressed:
Email: bushnelle@brandonu.ca
Phone: 204 571 7899
Page 1 of 23
https://mc06.manuscriptcentral.com/cjc-pubs
Canadian Journal of Chemistry

Draft
2
Abstract
Molybdenum is the only 4d-metal found in almost all life. One such molybdenum-containing
enzyme is sulfite oxidase which also contains the dithiolene-molybdopterin ligand. Sulfite
oxidase is essential in the degradation of sulfur containing compounds such as cysteine and
methionine. Past work has shown parallels in the chemistry of dithiolene- and diselenolene-metal
complexes. Thus, in this present work the O-atom transfer mechanism for a diselenolene sulfite
oxidase biomimetic complex was investigated using computational tools. The results of which
were compared to the analogous dithiolene biomimetic complex.
From the results obtained the molybdenum-diselenolene sulfite oxidase biomimetic complex is
able to catalyse the O-atom transfer and does so with a marginally lower value of ∆
r
G
‡
than that
for the analogous dithiolene complex. In particular, it was found that on average the diselenolene
complex had an activation energy 1.2 kJ mol
–1
lower in energy than the analogous dithiolene
complex. However, the calculated value of ∆
r
G suggests that the oxidation of sulfite is more
favourable for the dithiolene complex where the average difference in reaction aqueous Gibbs
reaction energy was -9.4 kJ mol
–1
relative to the diselenolene complex.
It is noted that the use of D3 and D3BJ corrections in combination with the B3LYP functional
the barrier for O-atom transfer is lowered by more than 30.0 kJ mol
–1
for both the diselenolene
and dithiolene complexes. Such results suggest that to study such oxo-transfer reactions the
proper treatment of dispersion interaction is necessary.
Keywords: Diselenolene, Density Functional Theory, Computational, Dithiolene, Sulfite
Oxidase Biomimetic Complex
Page 2 of 23
https://mc06.manuscriptcentral.com/cjc-pubs
Canadian Journal of Chemistry

Draft
3
Introduction
Molybdenum (Mo) is the only 4d-metal found in almost all life. In biological systems Mo
most often exists in the active site of an enzyme ligated by the molybdopterin (MPT) ligand;
notably, such enzymes are critical in carbon, nitrogen and sulfur metabolism.
1-4
The mononuclear
Mo-containing enzymes are divided into three main families; Dimethyl sulfide reductase
(DMSOr), xanthine oxidase and sulfite oxidase (SO) which are defined on the sequence and
structure of the oxidized active sites of the enzyme.
4
In general the mononuclear Mo-containing enzymes catalyse oxo-transfer reactions to or from
substrates. It is noted that an oxo-transfer reaction is defined such that: (i) an O-atom is
transferred and not O
2–
, (ii) the change in oxidation state of the metal is due solely to transfer of
the O-atom and (iii) the transferred O-atom is oxidic and is directly bound in a terminal or
bridging mode to the metal.
5
Previous DFT investigations have studied the oxo-transfer
mechanism for DMSOr biomimetic complexes.
6-7
One such study looked at two possible
mechanisms for the oxo-transfer between various mononuclear Mo complexes and various oxo-
atom acceptors/donors. Of the two possible pathways the preferred mechanism involves the lone
pair of electrons of the substrate attacking an oxo-ligand on the Mo via a 6-coordinate transition
state (TS). The alternative mechanism involves the formation of 7-coordinate intermediate
whereby the substrate first binds to the Mo center followed by an oxo-transfer to form product.
Importantly, the direct oxo-transfer (i.e., 6-coordinate TS) mechanism has a considerably lower
rate-determining barrier and is therefore the kinetically preferred pathway.
6
In another study the
oxo-transfer mechanisms between a biomimetic catalyst of DMSOr and several O-atom accepting
substrates were investigated.
7
In all cases a direct O-atom transfer occurs (i.e., 6-coordicate TS).
7
It is noted that in both of the above-mentioned studies the rate-limiting step is the O-atom transfer
step.
7-8
Page 3 of 23
https://mc06.manuscriptcentral.com/cjc-pubs
Canadian Journal of Chemistry

Draft
4
Due to the presence of one or more oxo-ligands highly oxidized metal centers are required. In
particular, the chosen metal cannot be in an oxidation state less than +4, with no more than 4 d-
electrons.
5
However, even with the highly oxidized metal center additional means are required to
stabilize this metal-oxo group. Such stabilization occurs through the folding of the MPT ligand.
9-
10
Importantly, the MPT ligand contains a dithiolene functional group which ligates the metal
center has been suggested to help tune different possible charges at the metal center.
11
In
particular, it is the ability of the dithiolene ligand(s) to fold that provides a mechanism for the
electronic buffering of the high positive charge on the metal center when it cycles between +4 to
+6 oxidation states.
4, 11-14
As mentioned above one enzyme that contains a MPT ligand is SO. This enzyme is essential
in the degradation of sulfur containing compounds such as cysteine and methionine.
13
Deficiencies in the enzyme activity often lead to premature death.
15-17
In particular it contains a
single MPT ligand that ligates the Mo center along with two oxide-oxygen (O
2–
) atoms and active
site cysteine residue.
17
It is noted that recent work has shown the parallels in the chemistry of
dithiolene- and diselenolene-metal complexes.
18-19
Thus, in this present work the O-atom transfer
mechanism is investigated to determine the aqueous Gibbs activation energy and the aqueous
Gibbs reaction energy for oxo-transfer by the diselenolene sulfite oxidase biomimetic complex.
In addition, the O-atom transfer mechanism is investigated for dithiolene sulfite oxidase
biomimetic complex to determine the effect of substituting the S with Se on the aqueous Gibbs
activation energies as well as the thermodynamics of the reaction.
Page 4 of 23
https://mc06.manuscriptcentral.com/cjc-pubs
Canadian Journal of Chemistry