Abstract: A new density functional (DF) of the generalized gradient approximation (GGA) type for general chemistry applications termed B97-D is proposed. It is based on Becke's power-series ansatz from 1997 and is explicitly parameterized by including damped atom-pairwise dispersion corrections of the form C(6) x R(-6). A general computational scheme for the parameters used in this correction has been established and parameters for elements up to xenon and a scaling factor for the dispersion part for several common density functionals (BLYP, PBE, TPSS, B3LYP) are reported. The new functional is tested in comparison with other GGAs and the B3LYP hybrid functional on standard thermochemical benchmark sets, for 40 noncovalently bound complexes, including large stacked aromatic molecules and group II element clusters, and for the computation of molecular geometries. Further cross-validation tests were performed for organometallic reactions and other difficult problems for standard functionals. In summary, it is found that B97-D belongs to one of the most accurate general purpose GGAs, reaching, for example for the G97/2 set of heat of formations, a mean absolute deviation of only 3.8 kcal mol(-1). The performance for noncovalently bound systems including many pure van der Waals complexes is exceptionally good, reaching on the average CCSD(T) accuracy. The basic strategy in the development to restrict the density functional description to shorter electron correlation lengths scales and to describe situations with medium to large interatomic distances by damped C(6) x R(-6) terms seems to be very successful, as demonstrated for some notoriously difficult reactions. As an example, for the isomerization of larger branched to linear alkanes, B97-D is the only DF available that yields the right sign for the energy difference. From a practical point of view, the new functional seems to be quite robust and it is thus suggested as an efficient and accurate quantum chemical method for large systems where dispersion forces are of general importance.


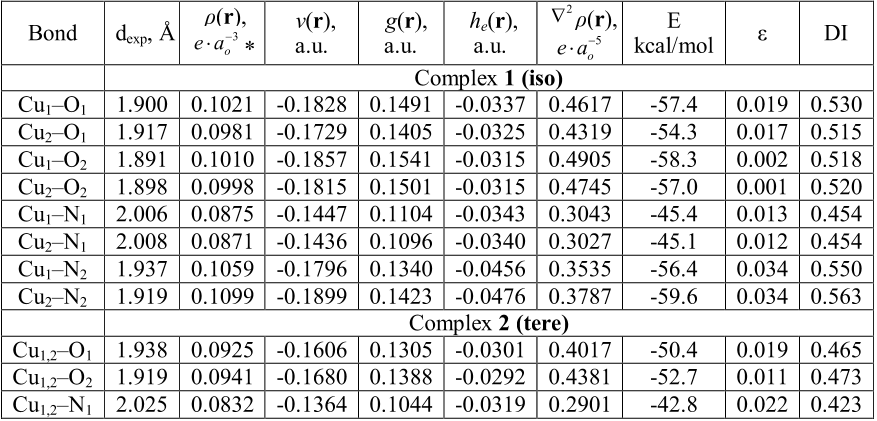
![Figure 3. Structure of the complexes 1–3 in accordance with X-ray data and QTAIM analysis. In terms of the QTAIM formalism [46], all the coordination Cu–N and Cu–O bonds should be assigned to the intermediate type interactions that are characterized by positive values of the electron density Laplacian r2 and the negative electron energy density he(r) values. From one aspect, the positive r2 values indicate outflow of electron density from interatomic space into the](/figures/figure-3-structure-of-the-complexes-1-3-in-accordance-with-x-3qrjud7v.png)