
University of Groningen
A computer-controlled continuous air drying and flask sampling system
Neubert, R.E.M.; Spijkervet, L.L.; Schut, J.K.; Been, H. ; Meijer, H.A.J.
Published in:
Journal of Atmospheric and Oceanic Technology
DOI:
10.1175/1520-0426(2004)021<0651:ACCADA>2.0.CO;2
IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
it. Please check the document version below.
Document Version
Publisher's PDF, also known as Version of record
Publication date:
2004
Link to publication in University of Groningen/UMCG research database
Citation for published version (APA):
Neubert, R. E. M., Spijkervet, L. L., Schut, J. K., Been, H., & Meijer, H. A. J. (2004). A computer-controlled
continuous air drying and flask sampling system.
Journal of Atmospheric and Oceanic Technology
,
21
(4),
651-659. https://doi.org/10.1175/1520-0426(2004)021<0651:ACCADA>2.0.CO;2
Copyright
Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the
author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons).
The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license.
More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne-
amendment.
Take-down policy
If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately
and investigate your claim.
Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the
number of authors shown on this cover page is limited to 10 maximum.
Download date: 10-08-2022

A
PRIL
2004 651NEUBERT ET AL.
q 2004 American Meteorological Society
A Computer-Controlled Continuous Air Drying and Flask Sampling System
R. E. M. N
EUBERT
,L.L.S
PIJKERVET
,J.K.S
CHUT
,H.A.B
EEN
,
AND
H. A. J. M
EIJER
Centrum voor IsotopenOnderzoek, Groningen, Netherlands
(Manuscript received 18 March 2003, in final form 21 November 2003)
ABSTRACT
A computer-controlled continuous air drying and flask sampling system has been developed and is discussed
here. This system is set up for taking air samples automatically at remote places. Twenty glass flasks can be
connected one by one or in pairs, and they can be filled at preset times, after preset intervals, or by online
remote control. The system is capable of drying air continuously without operator intervention, with a flow rate
of up to 4 L min
21
, to a dewpoint below 2508C. This enables continuous sampling, always retaining grab air
samples of, for example, the last 24 h. This way, it is possible to decide afterward, according to online instrument
records, if it is worthwhile to keep a single flask sample or even the whole diurnal cycle for later analysis at
the laboratory. Dry sample air can be supplied to other analyzers. Four copies of the instrumentation are active
at various places in Europe and have been shown to be able to run without servicing for periods of more than
1 month.
1. Introduction
Sampling of atmospheric whole air into glass flasks
for later laboratory analysis of trace gas concentrations
and isotopic ratios (commonly known as flask sampling)
has proven to be a tool of major importance in global
carbon cycle research (e.g., Conway et al. 1994; Keeling
et al. 1995; Francey et al. 1995). In this way, air samples
can be taken even at remote places with little infrastruc-
ture (and thus anthropogenic influences), providing bet-
ter observation coverage of larger areas.
The simplest way to take a flask sample is to evacuate
a flask in the laboratory, send it to the specific location,
and have it filled by an operator by just opening the
flask valve. Although this method is still successfully
applied in one of the global networks (Keeling et al.
1995), it has some distinct disadvantages.
1) After an extended period of storage under vacuum,
the inner surface of the glass flask is definitely not
in equilibrium with the air that suddenly flows in,
leading to several kinds of superficial de- and ad-
sorption processes after evacuation and sampling,
respectively, notably for CO
2
and its isotopomeres.
2) The air is not dried. This is unfavorable for the ox-
ygen isotopic ratio
18
O/
16
OinCO
2
, being sensitive
to oxygen atom exchange with traces of water (Gem-
ery et al. 1996), and it impairs O
2
/N
2
measurements
on the air.
Corresponding author address: Dr. R. E. M. Neubert, Centrum
voor IsotopenOnderzoek (CIO), University of Groningen, Nijenborgh
4, NL-9747 AG Groningen, Netherlands.
E-mail: neubert@phys.rug.nl
3) The sample quality depends critically on the vacuum
integrity of the flask seal. Even without a leakage,
there will be fractionating permeation going on
through the applied elastomere O-rings.
All these effects tend to be more of a concern with
lower flask volume. In the Keeling et al. (1995) network
that uses 5-L flasks, the disadvantages are still man-
ageable. However, for logistical reasons and the fact that
less sample air is needed nowadays due to advancements
in instrumentations, researchers strive for smaller sam-
ple flasks (down to 0.5 L). Unfortunately, the effects
mentioned above then deteriorate the sample quality to
an unacceptable point.
Thus, sampling strategies and techniques have
changed. Most networks now use preconditioned flasks;
that is, flasks filled with the appropriate pressure of dry
air that resembles the expected sample air as well as
possible in its analyzed constituents. These glass con-
tainers are filled in the field using a flushing device,
which flushes the air at the sampling place through the
flask for a certain period (15–30 min), after being dried
by a chemical drying agent [usually magnesium per-
chlorate, Mg(ClO
4
)
2
] or by using a cryogenic cold trap
if a power supply is available. The various networks
have constructed very straightforward ‘‘sampling suit-
cases,’’ with which a minimally trained technician can
easily and correctly perform this sampling procedure.
All this has complicated the situation somewhat, since
now some technical maintenance on the spot is neces-
sary, such as leak tests, pump and battery maintenance,
and above all frequent refreshment of the drying agent.
Typical examples are the National Oceanic and Atmo-
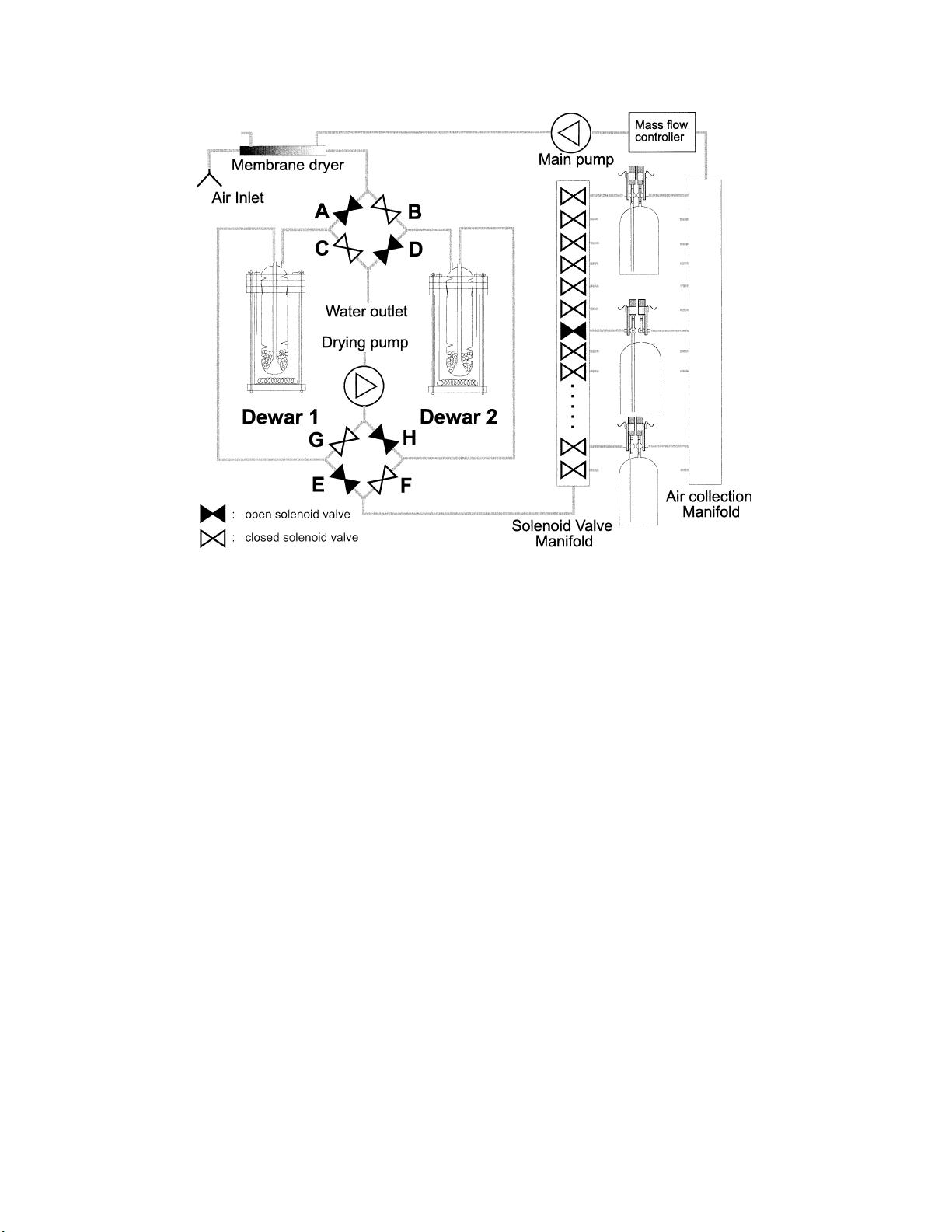
652 V
OLUME
21JOURNAL OF ATMOSPHERIC AND OCEANIC TECHNOLOGY
F
IG
. 1. Schematic drawing of the automatic flask sampling apparatus. After a predrying step (membrane
dryer close to the air inlet) one of the two cold traps dries the sample air to a dewpoint below 2408C,
while the other twin cold trap is regenerated at 1408C. The cryocoolers of the dewar vessels and the control
electronics are not shown here. The detailed working scheme is explained in the text.
spheric Administration/Climate Monitoring and Diag-
nostics Laboratory (NOAA/CMDL) AIRKIT for weekly
flask sampling at remote stations and their double-suit-
case aircraft sampler, capable of automatically sampling
flasks during one flight (Tans et al. 2001). The typical
sampling frequency for a flask sampling site is prefer-
ably once a week, or at least once every fortnight. De-
pending on how remote the actual location is, this re-
quires a significant amount of (traveling) time by the
person responsible for the sampling. Further require-
ments on the moment of sampling, such as a minimum
wind speed from a certain (clean air) wind sector or
hour of day interval, can normally only partially be met
by a person who can only devote part of his or her time
to this work (again depending very much on the travel
time that is required to the sampling place).
Another kind of study requires very frequent flask
sampling, for example, studies using diurnal cycle char-
acteristics (Zondervan and Meijer 1996; Meijer et al.
1996; Takahashi et al. 2002). Such sampling in diurnal
cycles even can be ‘‘continuous,’’ as in the first two
cited references above. That is, flask sampling goes on
continuously, refilling the same flasks every 24 h, until
an atmospheric condition, favorable for the specific ex-
periment, has occurred. The confirmation that such an
‘‘event’’ has occurred can only be drawn in hindsight
with the knowledge from online measurements. Thus,
the storage of the last 24 h of air in flasks at all times
is a necessity until confirmation is reached.
It is clear that an automated sampling apparatus sup-
plying dry air samples would have considerable advan-
tages, or would even be indispensable, for the purposes
mentioned above. We have built such an instrument. Up
to 20 flasks can be connected, a dry-air flow of up to
4 L min
21
can be supplied continuously, and the flasks
can be filled with either ambient atmospheric pressure
or with up to 150-kPa overpressure. Every flask is
closed (electrically actuated) with its own two O-ring
valves directly after flushing. The flask-filling schemes
are totally flexible, and an online connection via the
Internet or a modem and a mobile phone allows total
control over the system, as well as the monitoring of
all the critical parameters. This system is much more
sophisticated, after further development of the equip-
ment briefly described by Zondervan and Meijer (1996),
which did not meet the above-mentioned requirements.
2. The instrument
A schematic drawing of the system’s main parts is
shown in Fig. 1, and an overview photo of the instru-
mental arrangement is given in Fig. 2. Outside air is
sucked through the drying system (including a mem-
brane predrying step), through one or more of the 20

A
PRIL
2004 653NEUBERT ET AL.
F
IG
. 2. Pictorial overview of the complete installation: the valve manifolds are hidden on the backside of the solenoid valve frame; only
10 of possible 20 flasks are connected.
sample flasks that is opened, to the pump. The dry air
is then pumped out of the system again, and used as a
‘‘drying agent’’ in the predrying system. In the follow-
ing, we will discuss the design of the different parts,
namely, the drying concept, the airflow system includ-
ing the flasks, and the electronics and software, includ-
ing some remarks on installation tests. The valves in
this drawing (shown as double triangles) are open when
shaded black.
a. The drying concept
The system is designed to be able to dry, continuously
and unattended, an airflow of up to 4 L min
21
under all
meteorological conditions. This means that an automatic
recovery of the drying mechanism must be included,
and that during the recovery another means of drying
must be provided. We chose a double cryogenic drying
system as the most straightforward solution.
To freeze out the water vapor from the sample air,
we use cold traps made of glass (drawn in the dewar
vessels). They have an outer diameter of 5 cm and a
total length of 28 cm. The airflow is forced through the
entire cold trap from top to bottom, since the air exit is
a small glass tube, with its entrance close to the bottom
of the cold trap. The glass bodies have pencil-tip-like
indentations at the top and three rows of indentations
between 2 and 6 cm from the bottom to ensure turbulent
flow and good contact of the air with the cold-trap walls.
The lower 2.5 cm are filled with 3-mm-diameter glass
spheres to prevent ice crystals falling to the bottom from
being sucked through the center tube to the cold-trap
air outlet. The lower 15 cm of the cold traps are im-
mersed in a silicone-oil-based thermofluid (M60.115.05,
Renggli, Rotkreuz, Switzerland); each in a separate 2-
L stainless steel dewar vessel. A rubber-sealed plastic–
foam–plastic sandwich lid insulates the top of the dewar
from the outside air and facilitates all connections. The
closure of the lid is very important, in order to prevent
outside water vapor from entering the dewars, as it
would gradually form a ‘‘drop’’ of water (or ice, re-
spectively) at the bottom of the dewar and in between
the heating wires. With time, even a volumetric problem
would arise and make the thermofluid flow over. A Pt-
100 sensor measures the thermofluid temperature. The
fluid can either be cooled down to ø 2558Cbyanim-
mersion cryocooler probe (CC-65 II-R, Neslab Instru-
ments, Portsmouth, New Hampshire) or heated to
1408C by a resistance wire coil at the bottom of the
dewar. The glass cold traps are designed such that they
can take up to at least 75 g of water, which corresponds
to an airflow of 4 L min
21
with a relative humidity of
90% at 258C over a 15-h period.
At the inlet of the autosampler valve frame, there is
a cold-trap changeover valve setup, consisting of four
single solenoid valves [Fluid Automation Systems
(FAS), Versoix, Switzerland]. At a given time the sam-
ple air flows through valve A and cold trap 1 (with the
dewar vessel at 2558C) to another similar four-valve
installation and enters the solenoid valve manifold
through valve E. At the same time, a small membrane
drying pump (KNF Neuberger, Freiburg, Germany)
pumps room air through valve H, backward through cold
trap 2 (now heated to 1408C) and valve D to the water
outlet. In this way, water that was trapped in cold trap
2 in an earlier stage is removed. Theoretically, the dif-
ference between the absolute humidities in saturated air
at 408C and the laboratory air can be removed per unit

654 V
OLUME
21JOURNAL OF ATMOSPHERIC AND OCEANIC TECHNOLOGY
flushed air. However, condensation will occur in the
colder parts of the drying system and these water traces
will only be removed again with the dry warm air as
soon as the cold trap itself is dried. The energy con-
sumption of the solenoid valves keeps their body tem-
peratures during operation sufficiently high to prevent
condensation in the valves. In the following, cold trap
2 has to be prepared for drying again. Valves H and D
are closed, and dewar vessel 2 is cooled down to 2558C
again. During this process, the remaining humid air in
the tubing between valves H and F on the one side and
D and B on the other side (twice ø 0.5 m of tubing in
practice) is also effectively dried. The total regeneration
of a cold trap takes less than 12 h: 1 h heating, 9 h
flushing, and 1.5 h cooling down to 2408C (maximum
flushing temperature) is sufficient. Depending on the
environmental conditions, the cold-trap changeover
time can be extended to 18 h (our normal setting) or
even 24 h. This 18-h figure, combined with the moisture
capacity of the cold traps, makes the system suitable for
continuous dry air sampling in virtually every situation.
Still, it is favorable to remove a part of the water vapor
content already close to the air inlet of the system, es-
pecially if the inlet is far away from the autosampler,
as is the case for air inlets mounted on masts and towers.
The autosampler system is perfectly suited for the ad-
dition of a Nafion membrane predryer (MD 110-72-S,
Perma Pure, Toms River, New Jersey) close to the in-
take. A Nafion dryer consists of a polymer membrane
tube inside a stainless steel one. The membrane material
is only permeable for water vapor, which is actively
absorbed by sulfonic acid groups and moved along the
water vapor gradient. The incoming air passes through
the inner tube, while the volume between the inner and
outer tubes is flushed with dry gas in the opposite di-
rection to maintain the vapor gradient and remove the
water vapor to the waste outlet. This dry air is, in our
case, continuously supplied by the outlet of the auto-
sampler. Since the composition of this dry air is almost
identical to that of the inlet air (the dry air is actually
the inlet air from a short time before), the risk of influ-
encing the sample air composition due to eventual dif-
fusion processes through the membrane is minimized.
The dry air support to the Nafion dryer obviously re-
quires double tubing between the autosampler and the
inlet. In this arrangement, shown in Fig. 1 the air is
used as a drying agent flowing from the upper-right to
the upper-left connection, and the sample air from left
to right. The Nafion predryer removes between a half
and two-thirds of the water vapor content from the sam-
ple airstream, with the exact value depending on the
respective temperature and humidity (den Besten and
Neubert 1998). In setups with long inlet tubing, the
major advantage of using a Nafion predryer is to prevent
water vapor from condensing anywhere in the inlet line,
for example, if it is installed underground between a
tower and a laboratory building. We thereby exclude
the possibility of oxygen atomic (and thus also isotopic)
exchange between CO
2
and water close to or at the
condensation conditions (Gemery et al. 1996), which
might heavily alter the isotopic composition of atmo-
spheric CO
2
. The additional effect of lowering the water
vapor load of the drying system is also welcome, al-
though it is not strictly necessary, except under very
hot and humid sampling conditions.
The drying system has been extensively tested using
flows of heated air (to over 308C), and moisturized to
virtually 100% relative humidity. The final design of
the cold traps is able to effectively dry the air to a
dewpoint of ø2508C under all normal circumstances.
The moisture capacity of the cold traps is large enough
to make continuous operation possible (especially with
the assistance of the Nafion predryer). The drying sys-
tem is very robust and normally works error free for
several weeks without maintenance.
b. The airflow system and the flasks
The core of the airflow system is made of 6.35-mm
o.d. stainless steel tubing. The two 10-port solenoid
valve manifolds (distributing the air to the single flasks)
and the air collection manifold (collecting the air after
flushing through any one of the flasks) are custom made
from aluminium. The solenoid valves are of the same
type mentioned above. For connection purposes we use
vacuum-tight tube fittings (Swagelok, Solon, Ohio). The
tubing between the system and the flasks is 6.35-mm
Dekabon 1300 (Saint Gobain Performance Plastics,
Gembloux, Belgium), with 4.3-mm inner diameter and
a length of ø2 m per tubing. This is an aluminium
tubing, coated with a thin polyethylene layer on the
inside and a thick protective polyethylene tube on the
outside. We selected this material because it is robust
(very low risk of leak-causing damage) and yet flexible
and lightweight. Furthermore, it is easy to connect, and
thus a full set of 20 flasks can be exchanged in a short
time. Any influence on the composition of the trans-
ported air is virtually absent, as aluminium strongly re-
stricts diffusion into or out of the tubing. The relatively
large inner diameter accommodates flows of several li-
ters per minute over tens of meters with only a small
pressure gradient. Such a high flow is desirable, since
it minimizes the residence time of the air in the tubing
(in particular if the inlet point is far from the autosam-
pler). However, care must be taken to get the air into
thermal equilibrium with the room air before entering
the flasks (after all the air is cooled down to ø2558C),
as otherwise mass-dependent isotope (or gas type) frac-
tionation will occur.
The valve manifolds are made such that the dead
volumes are minimal. Preventing considerable dead vol-
umes (especially enclosing all tubing between manifold
and flasks), as well as the risk of one leaking flask
connection spoiling the whole series of samples, were
the main reasons to have a solenoid valve (manifold)
in addition to the flasks’ own electrically actuated




