This article has been accepted for publication and undergone full peer review but has not
been through the copyediting, typesetting, pagination and proofreading process which
may lead to differences between this version and the Version of Record. Please cite this
article as doi: 10.1002/jctb.6600
A continuous method for arsenic removal from groundwater using hybrid
biopolymer-iron-nanoaggregates: improvement through factorial designs
Marianela Batistelli
2
, Bárbara Pérez Mora
1
, Florencia Mangiameli
1,2
, Nadia Mamana
3
,
Gerardo Lopez
4
, María F. Goddio
4
, Sebastián Bellú *
1,2
and Juan C. González*
1,2
1
Área Química General e Inorgánica, Departamento de Química-Física, Facultad de
Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario, Suipacha
531, S2002LRK Rosario, Santa Fe, Argentina
2
Instituto de Química de Rosario-CONICET (IQUIR), Suipacha 570, S2002LRK
Rosario, Santa Fe, Argentina
3
Laboratorio de Materiales Cerámicos IFIR, CONICET, FCEIA, UNR, Bv. 27 de
Febrero 210 Bis, 2000 Rosario, Argentina.
4
NANOTEK SA, Parque Tecnológico Litoral Centro - Ruta Nacional 168, Argentina.
∗Corresponding author: Universidad Nacional de Rosario, Facultad de Ciencias
Bioquímicas y Farmacéuticas, Suipacha 531, S2002LRK Rosario, Santa Fe, Argentina.
Tel.: +54 341 4350214; e-mail addresses: gonzalez@iquir-conicet.gov.ar (Juan C.
González); bellu@iquir-conicet.gov.ar (Sebastián Bellú)
Abstract
This article is protected by copyright. All rights reserved.

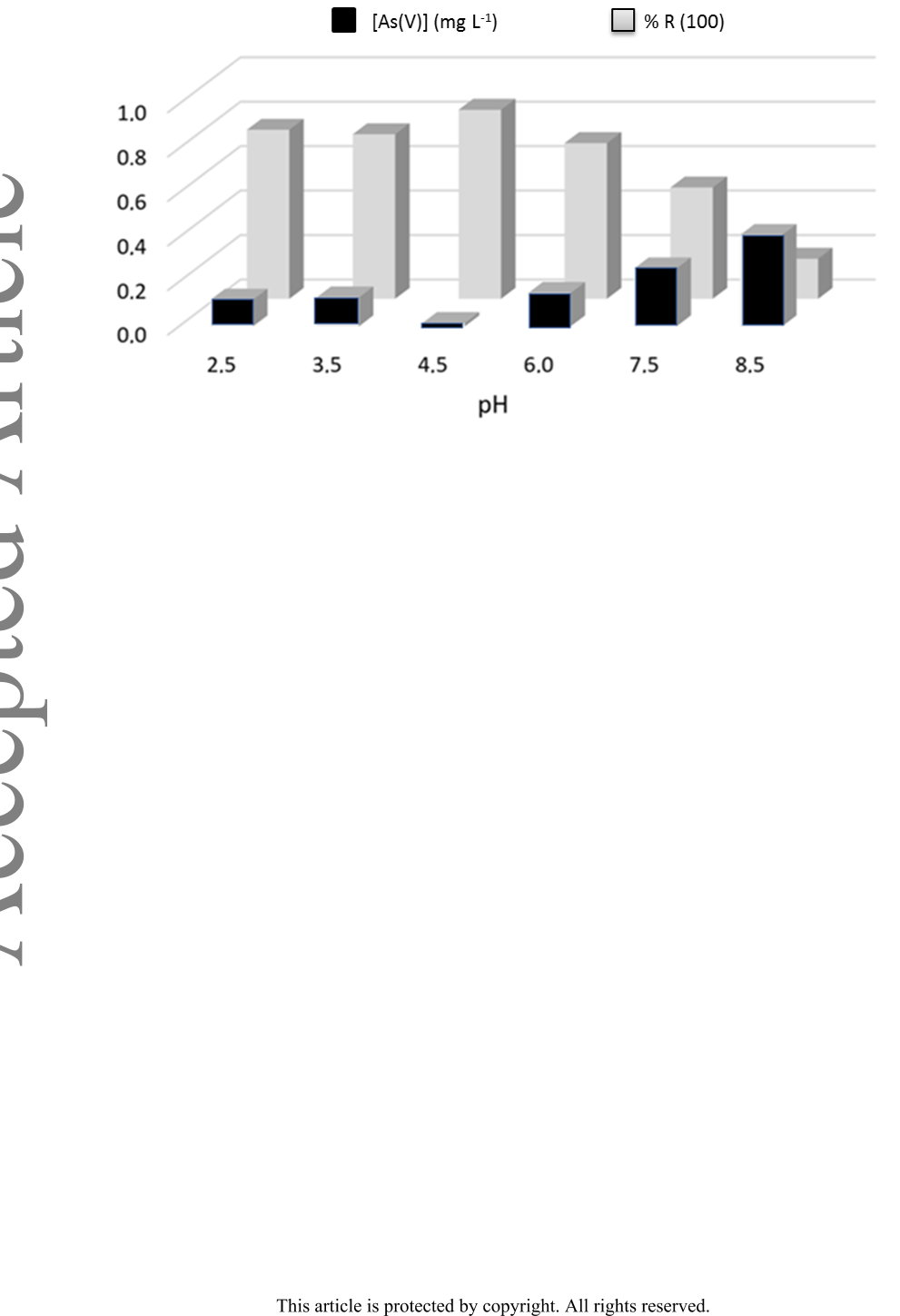
Background: Due to a variety of toxicological problems, the presence of As(V) in
aquifers is a significant problem. Sorption using chitosan doped with iron
nanoaggregates results in green and cheap methodology for its elimination.
Results: The hybrid sorbent was characterized by SEM, EDS, TGA, XRD, and FTIR
spectroscopy. Its stability against pH and time was determined by ICP-MS, while
conventional analytical techniques verified its Fe content. The sum of an individual
As(V) removal capacity by chitosan and iron nanoaggregates, was smaller than that of
the hybrid sorbent, indicating the existence of synergy.
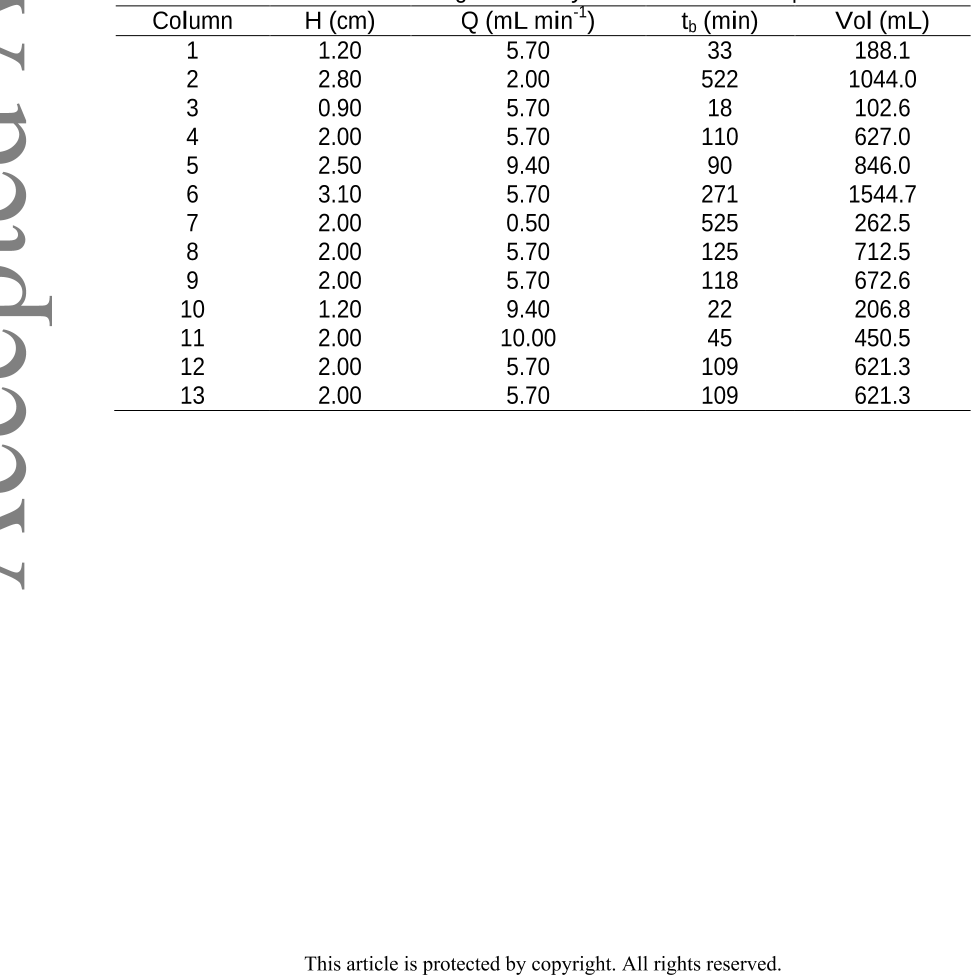
Conclusion: This study demonstrates the great capacity of the hybrid sorbent to
eliminate As(V) working with a continuous system (columns). The additional use of a
factorial design allows determining optimal operating values to optimize two responses.
In other words, in this multi-response system, column service time (tb) was minimized
and, at the same time, maximized the volumes of purified water obtained ([As(V)]
<0.05 m L
-1
) using desirability function.
Keywords: ARSENIC; IRON-NANOPARTICLE; GROUNDWATER;
IMPROVEMENT
1. Introduction
Arsenic (As) in water represents a global problem, affecting low- and high-income
countries
1
. More than 226 million people are exposed
2
through the ingestion of
contaminated drinking water and food
3
. Compounds containing As can be found in
This article is protected by copyright. All rights reserved.

soils, rocks and natural waters
4
. Both organic and inorganic forms are naturally found,
being the last the most toxic
5
. In natural waters, it is present with two predominant
oxidation states, As(III) and As(V)
6
.
Argentina is one of the most affected countries in Latin America
7
. In the Chaco-
Pampean plain areas, As concentrations vary in a wide range (0.005 - 5 mg L
-1
)
8
. About
50% of the population in rural areas is exposed to As poisoning, including several
clinical manifestations such as cancer, hypertension, diabetes, and hyperpigmentation
9
.
About 30% of the people exposed to As develop cancer, especially of skin and internal
organs
10
.
The guideline value of As in drinking water is 0.01 mg/L recommended by the World
Health Organization
11
, whereas the value of 0,05 mg/L, is valid for the Argentine
drinking water standards
12
.
For this reason, it is essential to develop a simple, economical, and sustainable As
removal technology. Most of the current methods to remove As include
oxidation/reduction, coagulation, precipitation, sorption, ionic exchange, membrane
technologies, and bioremediation
13
. However, some of these methods are expensive
and/or unfriendly to the environment. Biosorption is a novel method that has
demonstrated a high capacity to remove several contaminants
14-17
, mainly when applied
in continuous flow systems
16,18
. Our previous work showed chitosan capacity to remove
As from water and groundwater
16,19
. Chitosan doped with iron derived nanoparticles can
increase the adsorption properties of the material. This has been reported previously in
the literature
20-22
. Nanoparticles have high removal capacity and fast reaction kinetics
This article is protected by copyright. All rights reserved.

against contaminants due to their high surface/volume ratio. A combination of iron
nanoparticles and biopolymers increase the system stability, creating a synergy for As
removal
23
. The aim of this work was the synthesis of a new material based on chitosan
and iron derived nanopartiples (CIN) and its use in continuous treatment of natural
contaminated groundwater. The use of a factorial design allowed the determination of
the operating values to optimize two responses at the same time: minimum columns
service time (tb) and maximum purified water volume (Vol).
2. Experimental
2.1 Analytical methods
Groundwater natural samples were obtained from Piamonte Town, Santa Fe, Argentina.
Groundwater characterization was analized by standard methods and the results are
showed in Table S1. Water samples were supplemented by the addition of sodium
arsenate (Na
2
HAsO·7H
2
O) solution until 1.0 mg/L As(V). Arsenic concentration was
increased until 1.0 mg/L in column experiments in order to work with a value within the
concentration range found in natural groundwater of the area under study. All the
reagents for the current research were of analytical grade. As(V) quantification in
aqueous solutions was performed applying a self-made modification of molybdenum
blue method
16
. Detection Limit (DL) and Quantification Limit (QL) were 0.0043 mg L
-1
and 0.013 mg L
-1
, respectively. The molar extinction coefficient (ε) obtained in the
experimental linear range (0.0050-0.50 mg L
-1
) was (19150 ± 150) M
-1
cm
-1
.
2.2 Chitosan Iron Nanoaggegates synthesis (CIN)
This article is protected by copyright. All rights reserved.

Iron nanoparticles synthesis and stabilization was achieved from a stock solution
containing 1:2 molar ratio ferrous: ferric species, which was slowly poured (drop-wise)
into an alkali source, composed of sodium hydroxide, under vigorous stirring and
nitrogen sparging. Core-shell magnetic crystals (Feº core - magnetite and / or
maghemite shell) formed and precipitated. CIN was prepared by mixing a water-based
suspension of iron nanoaggregates, functionalized with starch to promote interaction
and linking to chitosan, incorporated as a powder. The mixture (1:20 w/w iron/chitosan)
was stirred to achieve homogenization. Then it was allowed to settle, supernatant water
was eliminated, and the material was dried at 60 °C for 72 hours. The resultant solid
was then ground to obtain a suitable powder.
Chitosan used for the CIN's synthesis was previously characterized (molecular weight
and deacetylation degree) by our group
15,19
.
2.3 CIN stability and total iron quantification
CIN stability was determined as follow: 1.0 g of CIN were mixed with 60.0 mL of acid
solution (pH 4.5 given by H
2
SO
4
) and stirred at 350 rpm for 4.5 h. The filtered
supernatant was analyzed to measure the presence of iron using ICP-MS. Quantification
of total iron was carried out by disaggregation of samples, and iron in aquous phase
were measured by ICP-MS.
2.4 pH zero-point charge (pH
ZPC
) determination
This article is protected by copyright. All rights reserved.