Vol. 25 No. 5 COST–BENEFIT ANALYSIS OF GOWNS 419
METHODS
Study Population
All patients staying more than 24 hours in a 19-bed
MICU at Barnes–Jewish Hospital from July 1, 1997, to
December 31, 1999, were eligible. All healthcare workers
and visitors were required to wear gowns and gloves on
entry into the rooms of patients colonized or infected with
VRE from July, 1, 1997, to June 30, 1998, and from July 1,
1999, to December 31, 1999. During the 12 months
between these two periods, gowns were not required. The
institutional review board committees of Saint Louis
University and Washington University approved this
study.
During the entire study period, all patients were
actively screened for VRE by collection of stool for cul-
tures or rectal swabs on admission, every 7 days, and at
discharge from the MICU. Per hospital protocol, stool
specimens sent for the detection of Clostridium difficile
toxin were also screened for VRE. For each patient with
VRE, a sign requiring contact precautions and an isolation
cart containing a dedicated stethoscope, a glass ther-
mometer, and gloves were placed at the entrance to the
patient’s room. Contact precautions were continued
unless a patient had two subsequent consecutive stool
surveillance specimens that tested negative. Gowns that
were fluid resistant and laundered after each use were
added to the isolation cart during the designated gown
periods.
A matched cohort study design was used to deter-
mine the attributable cost of VRE. Patients without VRE
from the same MICU population were matched to patients
with VRE by diagnosis-related group (DRG) code, Acute
Physiology and Chronic Health Evaluation (APACHE) II
16
severity of illness score (± 2 points), and age (± 5 years).
17
One patient without VRE was randomly selected for each
patient colonized with VRE when there were multiple
patients without VRE with the same matching criteria.
Two patients without VRE were randomly selected, using
the same matching criteria, for each patient with VRE bac-
teremia. Two matched controls were used to increase sta-
tistical power due to the small number of patients with
VRE bacteremia. Four patients colonized with VRE and
two patients with VRE bacteremia were excluded from the
study population because there was not a match of a
patient without VRE.
Clinical endpoints were obtained from the hospital’s
informatics system. These included MICU and hospital
lengths of stay, presence of nosocomial bacteremia due to
oxacillin-resistant S. aureus (ORSA) or Pseudomonas
aeruginosa, and presence of colitis or diarrhea associated
with C. difficile toxin. The three nosocomial pathogens
were used to determine whether the frequency of co-
infections was similar between patients with and patients
without VRE.
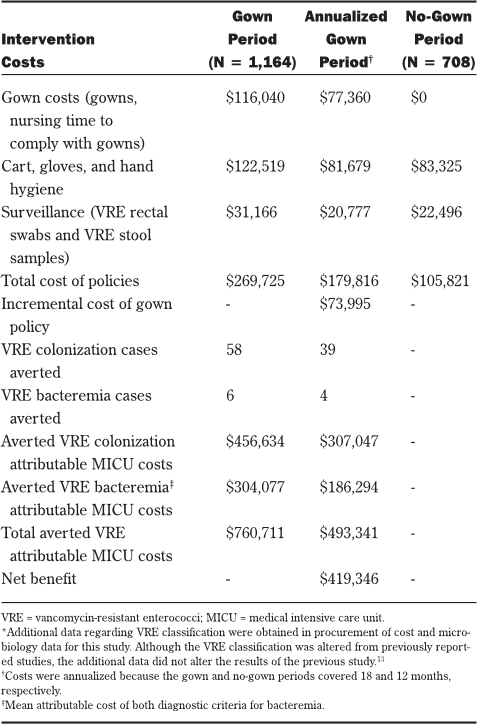
Costs
Overall costs for the VRE surveillance and infection
control program were estimated using the hospital’s step-
down cost allocation system, which recorded line-item
cost data per resource consumed and total cost per hospi-
tal admission. MICU costs were estimated from these
data by dividing the patient’s total hospitalization cost by
total days of hospitalization and then multiplying the quo-
tient by the patient’s total MICU-days. This data system
also provided hospital reimbursement data, type of insur-
ance, case mix index, and DRG. Medicare patients from
the study population were used to determine the average
non-reimbursed hospitalization cost by VRE status.
The cost for each isolation cart included all initial
supplies. In addition to the costs for gowns, the costs
resulting from staff time to comply with gown use were
estimated. Observational time trials were used to estimate
the time required for healthcare workers to retrieve, don,
doff, and properly dispose of gowns. On three separate
occasions, two unobtrusive observers measured the
amount of time required by 128 healthcare workers to
comply with the gown policy. Our observations showed
that the average worker needed 60 seconds (range, 35 to
95 seconds) to don and doff gowns, which was similar to
the amount of time needed for the same activities in anoth-
er study.
18
To estimate the cost associated with excess
workload per VRE patient contact, the average time was
multiplied by the average registered nurse salary (exclud-
ing fringe benefits). Because a range of healthcare work-
ers entered a patient’s room, the average registered
nurse’s salary was used to approximate this cost.
Microbiology costs for each patient were obtained
from line-item reports from the hospital’s microbiology
database. Microbiology costs were inclusive of all related
testing costs (ie, materials, technician time, nursing time
for culture procurement, and overhead). Individualized
costs associated with contact precautions and surveil-
lance are listed in Table 1. All costs were reported in U.S.
dollars.
Decision Analysis
An event pathway of the study was constructed
showing VRE colonization and infection rates during this
30-month study period (Figure).
13
Costs were allocated to
each arm based on actual resources consumed per
patient. Each patient with VRE, regardless of study peri-
od, was charged the costs for a cart, gloves, and hand
hygiene. During the gown period, patients with VRE were
charged additional costs for gowns and nursing time to
comply with the gown policy.
Benefits were measured as the number of VRE
cases and the MICU costs averted. The number of VRE
cases averted was estimated by multiplying the difference
in the VRE rates between the study periods by the num-
ber of patients in the gown period. The number of VRE
cases averted per 1,000 MICU-days was calculated by tak-
ing the number of cases averted and dividing it by the
total number of MICU patient-days in the gown period and
multiplying by 1,000. Averted attributable cost for the
gown period and net benefit of the gown policy
19
were
computed as shown in equations 1 and 2, respectively.