Q2. How much hydrogen coverage is attainable in the low trap density model?
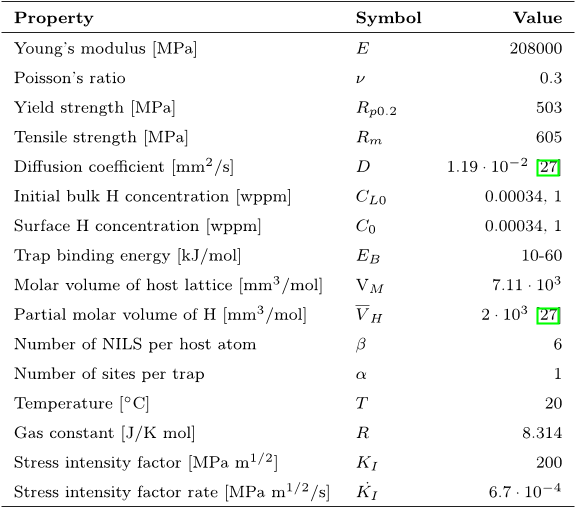
For the low trap density model, the maximum attainable trapped concentration of 0.033 wppm corresponds to a hydrogen coverage of 0.29 and a reduction in cohesive strength of 29 %.
Q3. What is the importance of assessing the hydrogen distribution in the material?
In order to predict the degrading effect of hydrogen on the mechanical properties, it is of fundamental importance to correctly assess the hydrogen distribution in the material.
Q4. What is the common method of implementing hydrogen influence into the cohesive model?
Most known attempts of implementing hydrogen influence into the cohesive model is through the HEDE principle [11, 15, 16, 58, 59, 60]; hydrogen reduction of the cohesive energy at fracture.
Q5. How much is the effective diffusivity ratio at the notch tip?
Assuming EB = 60 kJ/mol, the effective diffusivity ratio at the notch tip yield 0.62 and 0.005 for the low and high trap density models, respectively, at an initial concentration of 0.00034 wppm.
Q6. How can the authors determine the binding energies of trap sites?
Trap sites and trap binding energies can be established experimentally for a microstructure using varies approaches like electrochemical permeation or thermal desorption spectroscopy (TDS), with TDS considered best suited to provide detailed trap characteristics [5, 13, 23].
Q7. How many times an initial lattice concentration is 0.00034 wppm?
For the high trap density model, the maximum attainable trapped concentration is 10.1 wppm, 30000 times an initial lattice concentration of 0.00034 wppm.
Q8. How is the dislocation trapped hydrogen concentration calculated?
Using Equation (1) - (5), the dislocation trapped hydrogen concentration, CT , is calculated as a function of the lattice hydrogen concentration, CL, in terms of the trapping models by Kumnick and Johnson [2] and Sofronis et al. [34, 35], assuming VM = 7.106 · 10−6 m3/mol, β = 6, α = 1 and room temperature.
Q9. How can the authors estimate the hydrogen dependent cohesive stress for the fast separation case?
Using parameters representing of Fe (110); (2γint)0 = 4.86 J/m 2 and Γmax = 5.85 · 10−5 mol/m2 [55], assuming ∆g0i −∆g0s = 74.5 kJ/mol [13], the hydrogen dependent cohesive stress for the fast separation case can be estimated.
Q10. Why is austenite more diffusive than ferrite?
The substantially higher diffusivity in ferrite compared to austenite is due to the lower packing density of bcc metals, reducing the potential energy barrier for jumps.
Q11. What is the effect of the trap density on the effective diffusivity?
when the lattice concentration is increased from 0.00034 wppm to 1 wppm, maintaining a constant trap binding energy level, the effective diffusivity will increase.
Q12. What is the cleavage energy of Al(111) and Fe(110)?
An almost linear decrease in cleavage energy with increasing hydrogen coverage is observed for both Al(111) and Fe(110), as displayed in Figure 8b.
![Figure 3: Plot of the trapped hydrogen concentration (CT ) as a function of the lattice hydrogen concentration (CL), equivalent plastic strain (εp) and trap binding energy (EB). (a) Trapping model by Kumnick and Johnson [2]. (b) Trapping model by Sofronis et al. [34, 35].](/figures/figure-3-plot-of-the-trapped-hydrogen-concentration-ct-as-a-3tt1rt8b.png)
![Figure 8: Hydrogen effect on decohesion by quantum-mechanical approaches: (a) Traction separation curves for decohesion along Al(111) planes with a hydrogen coverage between 0 and 1, by Van der Ven and Ceder [54]. (b) Cleavage energy for decohesion along Al(111) and Fe(110) as a function of hydrogen coverage, by Jiang and Carter [55].](/figures/figure-8-hydrogen-effect-on-decohesion-by-quantum-mechanical-23kezcai.png)
![Figure 13: Normalized threshold stress intensity factor and normalized hydrogen dependent cohesive stress as a function of hydrogen concentration, according to experimental data by Thomas et al. [4], the linear decohesion model and the exponential decohesion model by Serebrinsky et al. [11], with ∆G0b = 30 kJ/mol.](/figures/figure-13-normalized-threshold-stress-intensity-factor-and-3c3wwpbe.png)
![Figure 4: Reported diffusion coefficients for hydrogen in iron and steel. Adapted from Grong [41].](/figures/figure-4-reported-diffusion-coefficients-for-hydrogen-in-d00c7l0u.png)

![Figure 7: Cohesive laws by Hillerborg et al. [47], Needleman [48] and Scheider [49].](/figures/figure-7-cohesive-laws-by-hillerborg-et-al-47-needleman-48-1ymh7zoe.png)
![Figure 11: Relationship between critical cohesive energy at fracture and hydrogen coverage for the polynomial cohesive law by Needleman [48]. Single hydrogen influence denotes hydrogen reduction of the critical cohesive stress. Double hydrogen influence denotes hydrogen reduction of the critical cohesive stress and of the critical separation.](/figures/figure-11-relationship-between-critical-cohesive-energy-at-bq8dksxp.png)
![Figure 12: Hydrogen influenced cohesive laws from the decohesion model by Liang and Sofronis [10], T 0n is the normal traction and q is a non-dimensional separation parameter.](/figures/figure-12-hydrogen-influenced-cohesive-laws-from-the-32g68x8i.png)
![Figure 10: Reduction in cohesive energy at different levels of hydrogen coverage for the polynomial cohesive law by Needleman [48], where (a) illustrates hydrogen influence on the cohesive strength only (single) and (b) illustrates hydrogen influence on both the cohesive strength and the critical separation (double).](/figures/figure-10-reduction-in-cohesive-energy-at-different-levels-3rbx36bt.png)
![Figure 2: Dislocation trap densities according to the work by Kumnick and Johnson [2] and the model by Sofronis et al. [34, 35]. In calculating CT , it is assumed αθT = 1, which accordingly gives the maximum possible hydrogen concentration trapped at dislocations.](/figures/figure-2-dislocation-trap-densities-according-to-the-work-by-bvfr3wyo.png)
![Figure 15: CTOD-R curves for various deformation rates, comparing experimental tests (symbol) and simulation results (lines) [14].](/figures/figure-15-ctod-r-curves-for-various-deformation-rates-3b0szsgq.png)
![Figure 9: Hydrogen coverage as a function of hydrogen concentration, for various levels of Gibbs energy (kJ/mol). Plotted according to the Langmuir-McLean isotherm [63].](/figures/figure-9-hydrogen-coverage-as-a-function-of-hydrogen-141pq59o.png)

