
LEISHMANIASIS
A sand fly salivary protein vaccine shows efficacy
against vector-transmitted cutaneous leishmaniasis
in nonhuman primates
Fabiano Oliveira,
1
Edgar Rowton,
2
Hamide Aslan,
1
* Regis Gomes,
1,3
Philip A. Castrovinci,
1
Patricia H. Alvarenga,
4,5
Maha Abdeladhim,
1
Clarissa Teixeira,
1,3
Claudio Meneses,
1
Lindsey T. Kleeman,
1
Anderson B. Guimarães-Costa,
1
Tobin E. Rowland,
2
Dana Gilmore,
1
Seydou Doumbia,
6
Steven G. Reed,
7
Phillip G. Lawyer,
2
John F. Andersen,
8
Shaden Kamhawi,
1†
Jesus G. Valenzuela
1†
Currently, there are no commercially available human vaccines against leishmaniasis. In rodents, cellular immunity
to salivary proteins of sand fly vectors is associated to protection against leishmaniasis, making them worthy targets
for further exploration as vaccines. We demonstrate that nonhuman primates (NHP) exposed to Phlebotomus
duboscqi uninfected sand fly bites or immunized with salivary protein PdSP15 are protected against cutaneous
leishmaniasis initiated by infected bites. Uninfected sand fly–exposed and 7 of 10 PdSP15-immunized rhesus ma-
caques displayed a significant reduction in disease and parasite burden compared to controls. Protection correlated
to the early appearance of Leishmania-specific CD4
+
IFN-g
+
lymphocytes, suggesting that immunity to saliva or
PdSP15 augments the host immune response to the parasites while maintaining minimal pathology. Notably,
the 30% unprotected PdSP15-immunized NHP developed neither immunity to PdSP15 nor an accelerated Leishmania-
specific immunity. Sera and peripheral blood mon onuclear cells from individuals naturally exposed to P. duboscqi bites
recognized PdSP15, demonstrating its immunogenicit y in humans. PdSP15 sequence and structure show no homol-
ogy to mammalian proteins, further demonstrating its potential as a component of a vaccine for human leishmaniasis.
INTRODUCTION
Leishmaniasis is a neglected tropical disease that affects the poorest of
communities and comes only second to malaria and fourth among
tropical parasitic diseases in mortality and morbidity, respectively (1).
Despite its global distribution and substantial disease burden, there
are no commercially available human leishmaniasis vaccines to date.
All forms of leishmaniasis are transmitted by the bite of infected
phlebotomine sand flies. As infected females feed on mammalian hosts,
they inject saliva, counteracting hemostasis and improving blood-
feeding success. Leishmania-infected sand flies regurgitate parasites
together with the salivary proteins into the bite wound. Exploiting the
concurrenceofsandflysalivaandparasitesinthebitesiteisanoriginal
approach to traditional Leishmania vaccines.
Experimentally, it has been shown that exposure to saliva through
bites of uninfected sand flies or immunization with an appropriate sal-
ivary protein protects rodents against cutaneous and visceral leishma-
niases (2–5). Saliva-mediated protection from leishmaniasis correlates
to the induction of a rapid sand fly saliva–specific T
H
1(Thelper1cell)
immune response at the bite site that steers the development of a faster
and more robust Leishmania-specific T
H
1 immunity with minimal pa-
thology (4, 6). Moreover, antibodies are not required for saliva-mediated
protection from leishmaniasis in murine models (3, 4).
Additionally, saliva-driven immunity protected against vector-transmitted
leishmaniasis (3, 4). This virulent mode of challenge, encompassing sand
fly saliva, promastigote secretory gel (7), and midgut-differentiated
Leishmania metacyclics, was shown to rescind the efficacy of a vaccine
established via needle challenge with Leishmania parasites (8), high-
lighting the robustness of saliva-mediated immunity to leishmaniasis.
Cutaneous leishmaniasis (CL) is the most widely distributed form of the
complex of diseases referred to as the leishmaniases. Annually, an estimated
0.7 million to 1.3 million new CL cases occur worldwide (9). Some two-
thirds of new CL cases occur in six countries including Afghanistan,
Algeria, Brazil, Colombia, Iran, and the Syrian Arab Republic (9). CL caused
by Leishmania major is prevalent in the Middle East, North Africa, and Sub-
Saharan Africa, where it is mainly transmitted by Phlebotomus papatasi or
Phlebotomus duboscqi sand flies (10). Here, we tested the capacity of ex-
posure to P. duboscqi uninfectedbitesorimmunizationwithits15-kD
salivary protein, PdSP15, in nonhuman prima te s (NHP) for prote ct io n
against vector-transmitted L. major. We uphold the concept of using
immunity to vector salivary proteins to protect humans from CL, dem-
onstrating their efficacy against vector-transmitted L. major in NHP.
RESULTS
Exposure to uninfected sand fly bites protects NHP against
sand fly–transmitted CL
To induce immunity to sand fly saliva in NHP, we exposed naïve rhesus
macaques to 20 P. duboscqi uninfected sand fly bites four times every
1
Vector Molecular Biology Section , Laboratory of Malaria and Vector Research, National
Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD
20852, USA.
2
Department of Entomology, Walter Reed Army Institute of Research, Silver
Spring, MD 20910, USA.
3
Centro de Pesquisas Gonçalo Moniz (CPqGM)–Fundação
Oswaldo Cruz (FIOCRUZ), Salvador, Bahia 40296-710, Brazil.
4
Laboratório de Bioquímica de
Resposta ao Estresse, Instituto de Bioquímica Médica, Universidade Federal do Rio de
Janeiro, Rio de Janeiro 21941-902, Brazil.
5
Instituto Nacional de Ciência e Tecnologia em
Entomologia Molecular (INCT-EM), Universidade Federal do Rio de Janeiro, Rio de Janeiro
21941-902, Brazil.
6
Faculty of Medicine, Pharmacy and Odontostomatology, University of
Bamako, Bamako 1805, Mali.
7
Infectious Disease Research Institute, Seattle, WA 98102,
USA.
8
Vector Biology Section, Laboratory of Malaria and Vector Research, National Institute
of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD 20852, USA.
*Present address: Nursing Department, Faculty of Health Science, Selahaddin Eyyubi
University, Diyarbakir, Turkey.
†Corresponding author. E-mail: jvalenzuela@niaid.nih.gov (J.G.V.); skamhawi@niaid.nih.gov (S.K.)
RESEARCH ARTICLE
www.ScienceTranslationalMedicine.org 3 June 2015 Vol 7 Issue 290 290ra90 1
on July 7, 2015Downloaded from

21 days. Most NHP (63%) developed a
delayed-type hypersensitivity (DTH) re-
sponse showing a marked recruitment
of mononuclear cells to the dermis 48 hours
after the last exposure (Fig. 1A). The re-
active bite site showed a relative abun-
dance of interferon-g (IFN-g)compared
to controls ascribing a T
H
1-like environ-
ment to the observed cell infiltrate (P =
0.0043, Mann-Whitney test; n =6;Fig.1B).
Exposure to uninfected sand flies also
generated a humoral response that was
more pronounced in DTH-positive reac-
tive animals (P = 0.0002, t test; n =10;
Fig. 1C). To test the efficacy of the observed
immunity to uninfected sand fly bites in
protection fro m Leishmania parasites,
we developed a natural model of vector-
transmitted CL in NHP using L. major–
infected P. duboscqi sand flies. Two groups
of NHP challenged with either 20 or 50
infected sand flies presented with 50 and
90% of diseased animals, respectively.
Lesion clusters evolved from papules to
nodules to ulcerated lesions, which pro-
gressed to healed scars (fig. S1), charac-
terist ics that mirror those observed in
human CL caused by L. major (11). Next,
we tested whether immunity generated
tosandflysalivaryproteinsinNHPwas
protective against vector-transmitted CL.
Naïve and uninfected sand fly–exposed
NHP were challenged with 50 Leishmania-
infected sand flies. Compared to naïve
animals, uninfected sand fly–exposed NHP
controlled the infection with a significant
reduction in disease burden, as defined
by the computation of the cumulative
measurement of the largest diameter of
each lesion on a weekly basis (P = 0.0083,
Mann-Whitney test; n =9to15;Fig.1D),
maximum lesion size (P = 0.0119, Mann-
Whitney test; n = 9 to 15; Fig. 1E), and
time to heal [P = 0.0048, log-rank (Mantel-
Cox) test; n =9to15;Fig.1F].Atthe
9-week post-challenge time point, 70%
of naïve animals displayed ulcerated le-
sions compare d to only 30% of uninfec ted
sand fly–exposedNHP(Fig.1G).Thisre-
duction in disease severity correlated to a
significantly lower number of parasites in
lesion biopsies (P = 0.0237, Mann-Whitney
test; n = 9 to 15;Fig. 1H). Notably, in order
not to disrupt the course of lesion develop-
ment, the parasite burden was measured
at 12 weeks after infection when les io n s
of both naïv e and uninfected sand fly–
ex p os e d NH P we r e he a l i n g . Furthermore,
Fig. 1. Exposure to P. duboscqi uninfected sand flies (USFs) induces an anti-saliva immunity that
protects NHP from vector-transmitted CL. (A to C) Immunity to USF bites 48 hours (A and B) or 2 weeks
(C) after the last exposure. (A) DTH response (left panel) and a hematoxylin and eosin–stained biopsy
section (right panel, ×400) from a USF bite site. (B) IFN-g mRNA expression in biopsies of a USF bite site
(Exp. Bites) or normal skin (Naïve) from the same animal. Biopsies were obtained from six randomly
selected bite site–reactive NHP (P = 0.0043, Mann-Whitney test; n = 6). (C) Anti-saliva immunoglobulin
G (IgG) levels before (Pre) or after (Post) exposure (P = 0.0002, t test; n =10).Cumulativedatafrom
two independent experiments are shown. OD, optical density. (D to K) Fifteen USF-exposed NHP (Exp.
Bites) and nine naïve NHP were challenged with 50 L. major–infected P. duboscqi. Cumulative data from
two independent experiments are shown. (D) Disease burden (P = 0.0083, Mann-Whitney test; n =9to15).
(E) Maximum lesion size (P = 0.0119, Mann-Whitney test; n = 9 to 15). (F) Kaplan-Meier plot of the healing
time, a cumulative measurement of lesion development from ulcer to scar [P = 0.0048, log-rank (Mantel-
Cox) test; n = 9 to 15]. (G) Representative photographs 9 weeks after challenge. (H) Parasite number
12 weeks after challenge (P = 0.0237, Mann-Whi tney test; n = 9 to 15). (I to K) PBMCs from seven naïve
(Naïve) and nine USF-exposed NHP (Exp. Bites) were stimulated with Leishmania antigen (Leish) 2 weeks
after challenge. Selection was based on cell number and viability. (I) IFN-g SFC by enzyme-linked immuno-
spot (ELISPOT) (P = 0.0587, Mann-Whitney test; n = 7 to 9). (J) Percent of CD4
+
IFN-g
+
lymphocytes by flow
cytometry (P = 0.0229, Mann-Whitney test; n = 7 to 9). (K) Frequency of CD4
+
IFN-g
+
lymphocytes
correlated to disease burden. Dashed line indicates 95% confidence interval (CI) (P = 0.0022, n = 16, Spearman
test r = −0 .72). Scale bar, 200 mm; lines and bars indicate the mean, and error bars indicate SEM.
RESEARCH ARTICLE
www.ScienceTranslationalMedicine.org 3 June 2015 Vol 7 Issue 290 290ra90 2
on July 7, 2015Downloaded from

protection in uninfected sand fly–exposed NHP correlated to the induc-
tion of an early Leishmania-specific immune response 2 weeks after
infection. After stimulation with Leishmania antigen (Leish), pe-
ripheral blood mononuclear cells (PBMCs) from uninfected sand fly–
exposed NHP produced a high er numbe r of IFN-g spot-forming cells
(SFC) compared to controls (P = 0.0587, Mann-Whitney test; n =7
to 9; Fig. 1I). We corroborated these data by flow cytometric analysis
demonstrating that CD3
+
cells were the main source of specific anti-
Leishmania IFN-g (fig.S2).AlthoughCD4
+
(Fig. 1J) and CD8
+
(fig.
S3A) lymphocytes produced Leishmania-specific IFN-g,onlythefre-
quency of CD4
+
IFN-g
+
cells was statistically higher between uninfected
sand fly–exposed and naïve animals (P = 0.0229, Mann-Whitney test;
n = 7 to 9; Fig. 1J). Moreover, the frequency of CD4
+
IFN-g
+
cells
inversely correlated to dise as e burd en in uni nfected sa n d fl y –exposed
NHP (P < 0.0022, n = 16, Spearman rank correlation test r = −0 .7211;
Fig. 1K and fig. S3B), suggesting that Leishmania-specific CD4 T cells are
participating in parasite clearance.
Reverse antigen screening of P. duboscqi sand fly salivary
molecules in saliva-exposed NHP identifies PdSP15 as a
vaccine candidate against CL
Having established that exposure to uninfected sand fly bites protects
NHP against CL, our next objective was to identify the salivary protein
responsible for the protective effect. Therefore, we screen about 23 se-
creted salivary proteins of P. duboscqi in NHP. To identify protective
sand fly salivary proteins while minimizing the number of NHP, we
developed an approach named “reverse antigen screening” (RAS). The
approach is based on exploiting the adaptive immunity generated against
salivary proteins in uninfected sand fly–exposed NHP. Uninfected
sand fly–exposed and bite site–reactive NHP were injected intrader-
mally with DNA plasmids coding for the most abundant secreted
P. duboscqi salivary proteins. Using the host machinery as a natural pro-
tein expression system, we selected salivary molecules that induced a
T
H
1-DTH 48 hours after inoculation. Salivary gland homogenate
(SGH) and bites from one uninfected sand fly, and empty plasmid were
used as positive and negative controls, respectively. From the 23 tested
DNA plasmids, we selected the top five molecules based on their in-
duction of the largest skin induration as measured by the diameter of
the skin reaction. These included PdMu54 (2.4 mm), PdSP15 (1.83 mm),
PdMu29 (1.79 mm), PdMu49 (1.69 mm), and PdMu35 (1.66 mm). We
also selected a negative control, empty DNA plasmid (1.01 mm), and
two positive controls, SGH (3.63 mm) and a bite site (2.84 mm) (Fig. 2A
and table S1). Of the five, PdSP15 was the only molecule displaying a
significant increase in IFN-g mRNA message compared to the ne gat i v e
control [P = 0.0109, one-way analysis of variance (ANOVA); n =8],and
the one exhibiting the lowest level of interleukin-4 (IL-4) (Fig. 2B);
this translated to a high IFN-g /IL-4 ratio indicative of a T
H
1-biased im-
mune response (P = 0.0470, one-way ANOVA; n =8;Fig.2C).Exact
P values for the five tested samples are presented in table S2.
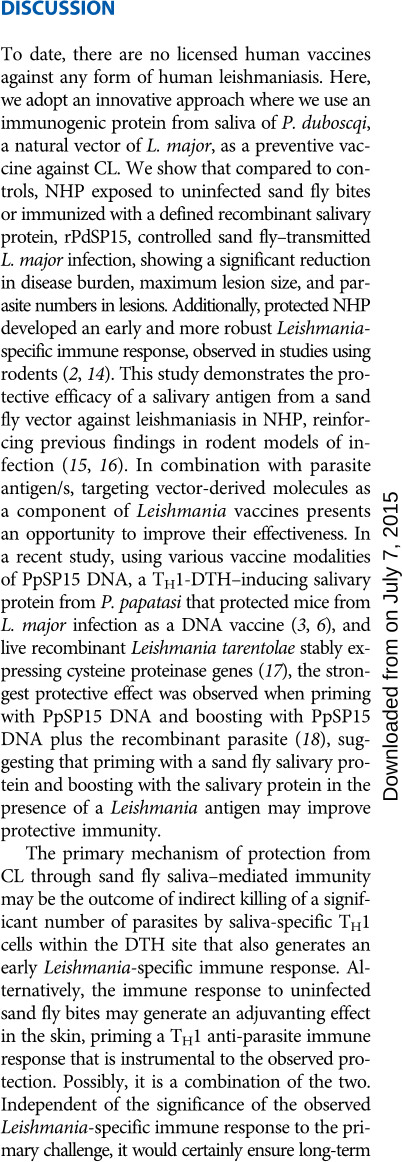
Immunization with PdSP15 protects NHP against sand
fly–transmitted CL
NHP were immunized intradermally with PdSP15 DNA two times 21
days apart and boosted 21 days later with recombinant PdSP15 (rPdSP15)
and glucopyranosyl lipid adjuvant in stable emulsion (GLA-SE). Con-
trol animals were inoculated with empty plasmid followed by a boost
with bovine serum albumin and GLA-SE. In contrast to controls, 70%
of PdSP15-immunized NHP displayed a distinct skin induration at the
injection site 48 hours after the rPdSP15 boost (P = 0.0067, Mann-
Whitney test; n = 10; Fig. 3A). Two weeks later, PBMCs of skin-reactive
PdSP15-immunized NHP produced significantly higher IFN-g SFC
after stimulation with rPdSP15 compared to controls (P = 0.0002, Mann-
Whitney test; n =7;Fig.3B,solidsquares).ThenumberofIFN-g SFC
in the 30% nonreactive PdSP15-immunized animals was similar to
controls (Fig. 3B, empty squares) but produced significantly high levels
of specific anti-rPdSP15 IgG antibodies (Fig. 3C, empty squares; P <
0.0001, one-way ANOVA; n = 3), with antibody levels showing a
negative cor relation to IFN-g production (P = 0.0037; r = –0.84,
Fig. 2. A RAS approach in NHP
identifies PdSP15 as a T
H
1-inducing
protein from saliva of the sand
fly P. duboscqi. NHP were exposed
three times to uninfected sand fly
bites. Two weeks after the last ex-
posure, animals were injected in-
tradermally with 23 distinct DNA
plasmids encoding the most abun-
dant P. duboscqi salivary proteins or an empty plasmid as a negative con-
trol. Bites from one sand fly or the inoculation of one pair of SGH was used
as positive controls. (A) Skin induration 48 hours after inoculation of plas-
mids measured using a Vernier caliper. Cumulative data of 14 NHP from
three independent experiments are shown. (B and C) Two-millimeter skin
biopsies of marked injection sites were obtained from 8 of 14 USF-exposed
NHP. (B) IFN-g and IL-4 mRNA expression by quantitative real-time fluores-
cence polymerase chain reaction (RT-qPCR). (C) IFN-g/IL-4 ratio for each
animal. Exact measurements and P values for all the samples tested are
presented in tables S1 and S2. Lines and bars indicate the mean, and error
bars indicate SEM.
RESEARCH ARTICLE
www.ScienceTranslationalMedicine.org 3 June 2015 Vol 7 Issue 290 290ra90 3
on July 7, 2015Downloaded from

Spearman correlation; n = 10; fig. S4A).
Because of the dichotomy of responses
to Pd SP 1 5 im mu niz atio n, we split PdSP15
vaccinated NHP to those that produced
IFN-g (PdSP15 –IFN-g
+
)orthosewhere
PdSP15 immunization induced a strong
antibody and a poor IFN-g resp onse
(PdSP15–IFN-g
−
). Control (CTL)- and
PdSP15-immunized NHP were challenged
with 50 L. major–infected sa n d fl i e s
1 month after the last immunization.
Compared to controls, PdSP15–IFN-g
+
(solid squares) NHP had significantly re-
d uced disease burden (P = 0.0490, one-
way ANOVA; n = 3 to 11; Fig. 3D) and reduced maximum lesion
size (P = 0.0465, one-way ANOVA; n = 3 to 11; Fig. 3E). PdSP15–IFN-g
−
NHP (empty squares) were not protected and had a disease burden
comparable to controls (Fig. 3, D and E). We did not observe a reduc-
tioninthetimetohealwhencomparingPdSP15–IFN-g
+
and controls
(Fig. 3F). Representative photographs illustrate the reduction in lesion
severity at 5 weeks after infection in PdSP15–IFN-g
+
NHP compared
to controls (Fig. 3G). Disease amelioration was further echoed by a
Fi g. 3. Immunization with PdSP15 pro-
tects NHP against vector-transmitted CL.
(A to C) Immunity in PdSP15- immunized
(PdSP15) or sham-immunized (CTL) NHP
48 hours (A) or 2 weeks (B and C) after last
immunization. (A) Skin induration after inoc-
ulation with bovine serum albumin (CTL) or
rPdSP15 (P = 0.0067, t test; n =10).(B)IFN-g
SFC by ELISPOT (P = 0.0002, t test; n =10).
(C) Anti-saliva IgG levels before (Pre) or after
(Post) immunization in controls (CTL), PdSP15-
immunized NHP producing IFN-g (PdSP15-
IFN
+
) or not (PdSP15-IFN
−
)(P < 0.0001, one-way
ANOV A; n =3to10).(D to L) Evaluation of
disease (D to H) and Leishmania-specific im-
munity (I to L) in CTL, PdSP15 -IFN
+
,or
PdSP15-IFN
−
NHP after challenge with 50
infected sand flies. (D) Disease burden (P =
0.0490, one-way ANOVA; n = 3 to 11). (E) Max-
imum lesion size (P = 0.0465, one-way ANOVA;
n = 3 to 11). (F) Kaplan-Meier plot of the
healing time [P = 0.1770, log-rank (Mantel-
Cox) test; n = 3 to 11]. (G) Representative
photographs 5 weeks after challenge. (H)
Parasite number 5 weeks after challenge (P =
0.0034, one-way ANOVA; n =3to8).(ItoK)
PBMCs stimulated with Leishmania antigen
(Leish) 2 weeks after challenge in 8 to 10 NHP.
Selection was based on cell number and vi-
ability. (I) IFN-g SFC by ELISPOT (P = 0.0075,
one-way ANOVA; n = 3 to 10). (J) Percent of
CD4
+
IFN-g
+
lymphocytes by flow cytometry
(P = 0.0002, one-way ANOVA; n =3to10).
(K) Frequency of CD4
+
lymphocytes produc-
ing cytokines (P = 0.0418, one-way ANOVA;
n = 4 to 6). (L) LST induration size 48 hours
after the injection of Leishmania antigen at
12 weeks after challenge (P = 0.0269, one-
way ANOVA; n = 3 to 10). Cumulative data
for 11 CTL and 10 PdSP15 NHP from two
independent experiments are shown. Lines
and bars indicate the mean, and error bars
indicate SEM.
RESEARCH ARTICLE
www.ScienceTranslationalMedicine.org 3 June 2015 Vol 7 Issue 290 290ra90 4
on July 7, 2015Downloaded from

significant reduction in the number of parasites in PdSP15–IFN-g
+
NHP compared to controls (P = 0.0034, one-way ANOVA; n =3to
8; Fig. 3H). PdSP15–IFN-g
−
NHP harbored parasite numbers compa-
rable to controls (Fig. 3H).
To understand how cellular immunity to PdSP15 protects against
vector-transmitted CL, we explored the early immune response to
Leishmania in PdSP15-immunized NHP 2 weeks after challenge with
infected sand flies. Similar to uninfected sand fly–exposed NHP, CD3
+
cells were the main source of specific anti-Leishmania IFN-g (fig.
S4B). Compared to controls, PdSP15–IFN-g
+
NHP (solid squares) de-
veloped a stronger anti-Leishmania immune response after challenge,
showing a significant increase in the number of IFN-g SFC (P = 0.0075,
one-way ANOVA; n = 3 to 8; Fig. 3I) and in the frequency of CD4
+
IFN-g
+
lymphocytes (P = 0.0002, one-way ANOVA; n = 3 to 10; Fig. 3J). No-
tably, a significant increase in the proportion of Leishmania-specific
CD4
+
IFN-g
+
IL-2
+
cells was also observed in PdSP15–IFN- g
+
NHP com-
pared to controls (P = 0.0418, one-way ANOVA; n = 4 to 6; Fig. 3K).
Similar to uninfected sand fly–exposed NHP, both CD8
+
and CD4
+
lymphocytes produced Leishmania-specific IFN-g, but only the fre-
quency of the latter was significantly higher in PdSP15–IFN-g
+
NHP
compared to controls (Fig. 3J and fig. S4C), reinforcing the conclusion
that the protective immune response is mostly driven by CD4
+
lym-
phocytes. This rapidly developing robust immunity against Leishmania
parasites was not observed in PdSP15–IFN-g
−
NHP (Fig. 3, I and J,
empty squares). Our findings suggest that PdSP15-specific IFN-g pro-
motes a microenvironment that facilitates priming of an early Leishmania-
specific protective CD4
+
T cell response.
In humans, the presence of a DTH after intradermal inocula-
tion with killed Leishmania, known as a positive Leishmanin skin
test (LST), is considered a signature of lifelong protective immunity
against CL. Twelve weeks after infection,
PdSP15–IFN-g
+
NHP had a significantly
larger LST induration size (Fig. 3L, solid
squares) compared to controls (P=0.0269,
one-way ANOVA; n = 3 to 11) and to
PdSP15–IFN-g
−
animals (Fig. 3L, empty
squares). This suggests that infected con-
trols and PdSP15 –IFN-g
−
NHP developed
a weaker immunity to Leishmania com-
pared to PdSP15–IFN-g
+
NHP after reso-
lution of the infection.
PdSP15 is a member of the
insect family of odorant-binding
proteins with no sequence or
structure homology to known
human proteins
The protective salivary antigen PdSP15
shares sequence homology only to the
small odorant-binding protein family
found exclusively in the salivary glands
of sand flies (Fig. 4A), with 67 and 54%
identity to the P. papatasi and Phlebotomus
sergenti salivary proteins PpSP15 and
PsSP15, respectively (Fig. 4B). To exclude
any structural similarities to human pro-
teins, the crystal structure of PdPS15 was
solved to a 2.95-nm resolution (Fig. 4C,
table S3). The structure is available at the Research Collaboratory
for Structural Bioinformatics Protein Data Bank (RCSB PDB) with
PDB code 4OZD. PdSP15 contains six a-helical elements designated
a, c, d, e, f, and g that match the homologous secondary structures of
insect odorant-binding proteins. Helix e is elongated relative to other
described insect proteins and contains a number of basic (arginine
and lysine) residues. Structural search with the program DALI (12)
showed a distant similarity to insect odorant-binding protein family
members including the D7 proteins found in the saliva of mosquitoes
and did not identify structural similarities to mammalian proteins
(fig. S5).
PdSP15 is immunogenic in humans naturally exposed to
P. duboscqi bites
Having established that PdSP15 is an antigen foreign to humans, we
investigated the immunogenicity of rPdSP15 in individuals naturally
exposed to P. duboscqi bites (13). Sand fly–exposed individuals with
antibodies to whole saliva produced significant levels of antibodies
to rPdSP15 (P < 0.0001, Mann-Whitney test; n = 12 to 30; Fig. 5A) or
the SGH (P < 0.0001, Mann-Whitney test; n =12to30;Fig.5A).PBMCs
from 14 individuals naturally exposed to P. duboscqi bites (18 to 65 years
old) were stimulated with SGH or rPdSP15 in vitro, and supernatants
were collected 96 hours after stimulation. Levels of IFN-g, IL-10, IL-17,
IL-5, and IL-13 were detected by a Luminex assay (Fig. 5B). Levels of
IL-2, IL-4, and IL-9 in these samples were below the limit of detection
of the assay. Compared to controls, stimulation with SGH induced sig-
nificant levels of IFN-g (mean, 294.6 pg/ml; P = 0.0354, one-way
ANOV A; n = 14; Fig. 5B), IL-10 (mean, 32.47 pg/ml; P = 0.0112, one-
way ANOVA; n = 14; Fig. 5B), IL-17 (mean, 245.4 pg/ml; P = 0.0004, one-
way ANOVA; n = 14; Fig. 5B), and IL-5 (mean, 65.27 pg/ml; P = 0.0344,
Fig. 4. PdSP15 is an odorant-binding protein in saliva of phlebotomine sand flies. (A) Phylogenetic
tree analysis shows the similarity of odorant-binding proteins in New and Old World sand fly species and
their divergence from odorant-binding proteins (OBP) of other dipterans and humans. Bootstrap value,
10,000. PdSP15 location is underlined in red. (B) Sequence alignment between PdSP15 from P. duboscqi
(accession number 112361953) and its orthologs in P. papatasi (PpSP15, accession number 449060564)
and P. sergenti (PsSP15, accession number 299829414). Black shading and gray shading represent identical
and similar amino acids, respectively. (C) Crystal structure of PdSP15 (4OZD) containing six a-helical
elements designated as a, c, d, e, f, and g.
RESEARCH ARTICLE
www.ScienceTranslationalMedicine.org 3 June 2015 Vol 7 Issue 290 290ra90 5
on July 7, 2015Downloaded from
![Fig. 3. Immunization with PdSP15 protects NHP against vector-transmitted CL. (A to C) Immunity in PdSP15- immunized (PdSP15) or sham-immunized (CTL) NHP 48 hours (A) or 2 weeks (B and C) after last immunization. (A) Skin induration after inoculation with bovine serum albumin (CTL) or rPdSP15 (P = 0.0067, t test; n = 10). (B) IFN-g SFC by ELISPOT (P = 0.0002, t test; n = 10). (C) Anti-saliva IgG levels before (Pre) or after (Post) immunization in controls (CTL), PdSP15immunized NHP producing IFN-g (PdSP15IFN+) or not (PdSP15-IFN−) (P < 0.0001, one-way ANOVA; n = 3 to 10). (D to L) Evaluation of disease (D to H) and Leishmania-specific immunity (I to L) in CTL, PdSP15-IFN+, or PdSP15-IFN− NHP after challenge with 50 infected sand flies. (D) Disease burden (P = 0.0490, one-way ANOVA; n = 3 to 11). (E) Maximum lesion size (P = 0.0465, one-way ANOVA; n = 3 to 11). (F) Kaplan-Meier plot of the healing time [P = 0.1770, log-rank (MantelCox) test; n = 3 to 11]. (G) Representative photographs 5 weeks after challenge. (H) Parasite number 5 weeks after challenge (P = 0.0034, one-way ANOVA; n = 3 to 8). (I to K) PBMCs stimulated with Leishmania antigen (Leish) 2 weeks after challenge in 8 to 10 NHP. Selection was based on cell number and viability. (I) IFN-g SFC by ELISPOT (P = 0.0075, one-way ANOVA; n = 3 to 10). (J) Percent of CD4+IFN-g+ lymphocytes by flow cytometry (P = 0.0002, one-way ANOVA; n = 3 to 10). (K) Frequency of CD4+ lymphocytes producing cytokines (P = 0.0418, one-way ANOVA; n = 4 to 6). (L) LST induration size 48 hours after the injection of Leishmania antigen at 12 weeks after challenge (P = 0.0269, oneway ANOVA; n = 3 to 10). Cumulative data for 11 CTL and 10 PdSP15 NHP from two independent experiments are shown. Lines and bars indicate the mean, and error bars indicate SEM.](/figures/fig-3-immunization-with-pdsp15-protects-nhp-against-vector-ntwxnkdh.png)
