A study of the T2 defect and the emission properties of the E3 deep level in
annealed melt grown ZnO single crystals
W. Mtangi, M. Schmidt, F. D. Auret, W. E. Meyer, P. J. Janse van Rensburg et al.
Citation: J. Appl. Phys. 113, 124502 (2013); doi: 10.1063/1.4796139
View online: http://dx.doi.org/10.1063/1.4796139
View Table of Contents: http://jap.aip.org/resource/1/JAPIAU/v113/i12
Published by the American Institute of Physics.
Additional information on J. Appl. Phys.
Journal Homepage: http://jap.aip.org/
Journal Information: http://jap.aip.org/about/about_the_journal
Top downloads: http://jap.aip.org/features/most_downloaded
Information for Authors: http://jap.aip.org/authors
Downloaded 06 May 2013 to 137.215.6.53. This article is copyrighted as indicated in the abstract. Reuse of AIP content is subject to the terms at: http://jap.aip.org/about/rights_and_permissions

A study of the T2 defect and the emission properties of the E3 deep level
in annealed melt grown ZnO single crystals
W. Mtangi,
1
M. Schmidt,
1
F. D. Auret,
1
W. E. Meyer,
1
P. J. Janse van Rensburg,
1
M. Diale,
1
J. M. Nel,
1
A. G. M. Das,
2
F. C. C. Ling,
3
and A. Chawanda
4
1
Department of Physics, University of Pretoria, Private Bag X20, Hatfield 0028, South Africa
2
School of Information Technology, Monash South Africa, Roodepoort 1725, South Africa
3
Department of Physics, University of Hong Kong, Pokfulam Road, Hong Kong
4
Department of Physics, Midlands State University, P. Bag 9055, Senga Road, Gweru, Zimbabwe
(Received 2 November 2012; accepted 7 March 2013; published onl ine 22 March 2013)
We report on the space charge spectroscopy studies performed on thermally treated melt-grown
single crystal ZnO. The samples were annealed in different ambients at 700
C and also in oxygen
ambient at different temperatures. A shallow donor with a thermal activation enthalpy of 27 meV
was observed in the as-received samples by capacitance-temperature, CT scans. After annealing the
samples, an increase in the shallow donor concentrations was observed. For the annealed samples,
E27 could not be detected and a new shallow donor with a thermal activation enthalpy of 35 meV
was detected. For samples annealed above 650
C, an increase in acceptor concentration was
observed which affected the low temperature capacitance. Deep level transient spectroscopy
revealed the presence of five deep level defects, E1, E2, E3, E4, and E5 in the as-received samples.
Annealing of the samples at 650
C removes the E4 and E5 deep level defects, while E2 also
anneals-out at temperatures above 800
C. After annealing at 700
C, the T2 deep level defect was
observed in all other ambient conditions except in Ar. The emission properties of the E3 deep level
defect are observed to change with increase in annealing temperature beyond 800
C. For samples
annealed beyond 800
C, a decrease in activation enthalpy with increase in annealing temperature
has been observed which suggests an enhanced thermal ionization rate of E3 with annealing.
V
C
2013
American Institute of Physics.[http://dx.doi.org/10.1063/1.4796139]
I. INTRODUCTION
ZnO is a wide and direct bandgap semiconductor with a
high exciton binding energy, high saturation velocity, high
electron mobility, and excellent resistance to radiation dam-
age. The latter feature of the material qualifies it for the fabri-
cation of devices that can operate in high radiant conditions,
e.g., in outer space applications and reactor laboratories.
Since in space applications, devices are usually subjected to
very harsh temperature conditions, it is a requirement for the
material to be resistant to high temperature annealing effects
for better operating efficiency and lifetime of devices. This is
because annealing tends to modify the operation of devices
by inducing defects, recovering/activating the neutral dop-
ants, increasing the concentration of shallow donors
1,2
result-
ing in surface conduction,
3
and at times annealing out
defects. For devices operating in the UV region, the problem
arises when defects induced in the material due to high tem-
perature exposure are optically active. Optical absorption by
these defects will influence efficient operation of devices.
Considering the low oxygen and nitrogen partial pres-
sures as well as the high temperatures which a detector on a
space craft is exposed to, there is a need to have knowledge
of the defects that can be introduced at high temperatures
under different ambient conditions. Studies on the annealing
induced defects in ZnO have been performed using deep
level transient spectroscopy (DLTS) techniques in which
deep level defects have been observed. It has also been dem-
onstrated that at some particular temperatures, formation of
these deep level def ects is ambient related. Quemener et al.
4
have investigated the effects of annealing hydrothermally
grown ZnO samples at 1100
C in Ar, O
2
, and Zn. Their
results have revealed the introdu ction of E2 whose formation
strongly depends on the ambient used. Mtangi et al.
5
also
investigated the effects of annealing melt grown single crys-
tals at 300
C in Ar, O
2
, and H
2
and they have revealed the
introduction of E4 in Ar and H
2
ambient. Annealing ZnO at
1100
C in Zn vapour and Ti has been performed by Weber
and Lynn.
6
Selim et al.
7
have also investigated the effects of
annealing ZnO under Zn-rich conditions and reported a red
colouration of ZnO which has been attributed to O
v
, while
Ehret and Greenstone
8
have also reported the changes in the
colour of red ZnO upon heat treatment at different tempera-
tures, a hint which points out to the deep level defects that
are formed at those temperatures. Clearly, the effects of ther-
mal treatm ent have an effect on the optical and electronic
properties of ZnO.
Schmidt et al.
9
have demonstrated the photo-ionisation
of a defect level T2 observed in Pulsed Laser deposited
grown ZnO samples using optical deep level transient spec-
troscopy (ODLTS) while Ellguth et al.
10
showed the photo-
ionisation of the T2 and E4 defects in PLD ZnO thin films
also using ODLTS. Such observation has not been reported
in bulk single crystal ZnO and neither has T2 been observed
in as-grown Cermet single cry stal ZnO. Current literature
indicates that the T2 has only been observed in polycrystal-
line ZnO, thin films, and also in vapour phase grown material
used by Frank et al.
11
Ye et al.
12
have reported a defect with
0021-8979/2013/113(12)/124502/8/$30.00
V
C
2013 American Institute of Physics113, 124502-1
JOURNAL OF APPLIED PHYSICS 113, 124502 (2013)
Downloaded 06 May 2013 to 137.215.6.53. This article is copyrighted as indicated in the abstract. Reuse of AIP content is subject to the terms at: http://jap.aip.org/about/rights_and_permissions

the same activation enthalpy as T2 after O-implantation and
subsequent annealing of melt grown ZnO samples in air,
while Mtangi et al.
13
reported a defect they labelled Ex with
a similar activation enthalpy to that of T2 after annealing
melt grown single crystal ZnO samples at 700
CinArþ O
2
.
It must be noted that the material used by Ye et al.
12
and
Mtangi et al.
13
was obtained from the same supplier. Frank
et al.
11
also demonstrates that the E4 is optically active and
is a negative-U centre. Therefore, the existence of the T2/Ex
and E4 defects in ZnO crystals will definitely affect device
operation within the UV region.
Also of interest in ZnO defect studies is a defect labelled
E3. This defect has been observed to be present in all ZnO
crystals in very high concentrations regardless of material
growth technique. The study of this defect and its identity is
an interesting topic as it is not clear where it originates from
as yet. Research performed on ZnO, however, has the vast
majority of reports assigning it to an oxygen vacancy related
defect,
14–16
while other reports suggest it to be a transition
metal ion related defect.
17,18
Debate about the identity of E3
is still raging. With the difficulty in growing reproducible
p-type ZnO, knowing the identity of E3 and its behaviour,
i.e., in terms of its annealing behaviour can be essential as
this might assist in controlling material doping. It has since
been observed that the conditions under which T2 and E4 are
introduced reduce the intensity of the E3 peak.
13
Using the capacitance based DLTS technique, under dark
conditions, it has been observed from the DLTS spectra that
E3 has a peak height which increases with increase in rate
window frequency as has been reported by,
12,19
an indication
that it has a temperature activated capture cross-section.
However, its capture barrier energy has not been obtained yet
as its capture process seems not to follow a simple exponen-
tial process. It was also suggested that the electron capture
rate for the E3 level is not constant during the filling pulse as
it depends on the occupancy of the defect and the available
carriers for electron capture.
19
In this paper, we perform a further investigation on the
introduction of T2/Ex in ZnO under various annealing condi-
tions and the effects of high temperature oxygen annealing
on it using the conventional DLTS measurement techniques.
We also report, for the first time, on the observed change in
emission properties and activation enthalpy of the E3 defect
with changes in annealing temperature.
II. EXPERIMENT
Melt grown single crystal ZnO samples obtained from
Cermet Inc. with a batch number K3-1553-01 AV were used
in this study. Six sa mples were cut from the same 1 cm
2
wa-
fer. The as-received sample was used as the reference sample
for this experiment. The rest of the samples were then
annealed at 700
C for 1 h in different ambient conditions.
Before annealing, the samples were ultrasonically cleaned in
methanol for 5 min and blow dried using nitrogen gas. For
Ar þ O
2
annealing, a set of two samples was first annealed in
Ar. One of the samples was then removed from the furnace.
The remaining sample was then annealed for another 1 h in
O
2
ambient. For the O
2
þ Ar annealing, a set of two samples
was also used. First the two samples were annealed in O
2
, af-
ter which one of the samples was removed. The remaining
sample was annealed in Ar ambient for another 1 h. Vacuum
annealing was performed at a pressure of approximately
1 10
6
Torr. For annealing involving Ar and O
2
, a con-
trolled flow of gas of 3.0 l/min was used. For samples
annealed in O
2
ambient at different temperatures, a 1 cm
2
wafer from the same batch of samples as in the latter case
was used. All annealing was performed prior to Schottky and
ohmic contact fabrication. Prior to contact fabrication, all
samples were cleaned as outlined by Mtangi et al.
13
Pd
Schottky contacts of diameter 0.5 mm and thickness 50 nm
were fabricated on the Zn-polar face while ohmic contacts of
Al/Au with thicknesses of 30/50 nm were fabricated on the
O-polar face of the samples. Contact fabrication was done
using the resistive evaporation system at a pressure of
approximately 1 10
6
Torr.
III. RESULTS AND DISCUSSIONS
From the IV characteristics of the Schottky contacts
fabricated on the ZnO samples used in this study, ideality
factors of 1.09–1.40 were calculated. This indicates that pure
thermionic emission proved to be the dominant current trans-
port mechanism at room temperature. The as-received sam-
ple showed a leakage current in the order of 10
9
Aata
reverse bias of 2.0 V and a series resistance in the order of
600 X. For the annealed samples, the oxygen annealed sam-
ples produced contacts with the least leakage current values
in the order of 10
10
A at a reverse bias of 2.0 V and series
resistance values in the order of 3 k X, while the vacuum
annealed samples gave contacts with high leakage currents,
in the order of 10
6
A at 2.0 V and series resistance values
which are lower than those obtained in the as-received
samples. Barrier heights of 0.82 eV were measured on the
contacts fabricated on the oxygen annealed samples, while
lower barrier heights of 0.66 eV were measured for the vac-
uum annealed samples. All the contacts fabricated were suit-
able for use in space charge spectroscopic techniques.
A. Shallow donors
We have investigated the effects of annealing on the
shallow donors and the net doping concentration of the
samples using capacitance temperature (C-T) scans and
capacitance-voltage (C-V) measurements (van Opdorp’s
method
20
), respectively. In Figure 1(a), the net-doping con-
centration of the samples annealed at 700
C in different
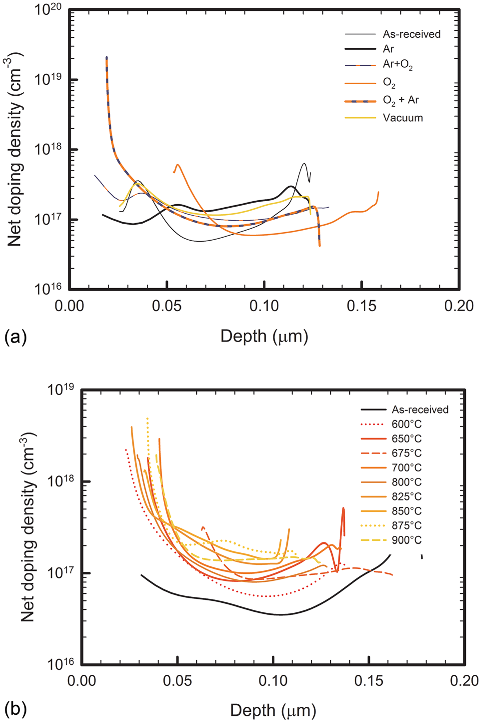
ambients is presented. From the net doping profiles presented
in Figure 1(a), we observed that the net doping concentration
increases for all the annealed samples. In ambients with low
oxygen partial pressures (Ar and vacuum), the net doping
concentration is higher within a depth of 0.10 lm below the
interface compared to that of the as-received sample.
For the samples annealed in oxygen ambient at different
temperatures (Figure 1(b)), the net doping concentration for
all the annealed samples is observed to increase as compared
to that of the as-received samples. This increase is observed
to be highly pronounced towards the surface. At a depth of
0.10 lm below the interface, an increase in net doping density
124502-2 Mtangi et al. J. Appl. Phys. 113, 124502 (2013)
Downloaded 06 May 2013 to 137.215.6.53. This article is copyrighted as indicated in the abstract. Reuse of AIP content is subject to the terms at: http://jap.aip.org/about/rights_and_permissions

with annealing is observed up to a temperature of 675
C,
after annealing at 700
C and 800
C, there is a decrease in
the net doping density. An increase in net doping density is
again noticed after annealing between 825
C–875
C and a
decrease is observed after annealing at 900
C. The C-T meas-
urements in Figure 2(b) can be used to explain this trend,
since the C-V measurements were performed at 300 K, where
a fluctuation in the capacitance values is also observed. This
fluctuation can be attributed to the partial contribution of the
E3 level to the capacitance at this temperature which influen-
ces the determination of the net doping concentration. For
samples annealed above 650
C, this effect is crucial since the
slope in the C-T characteristics at 300 K is very steep.
For the C-T scans presented in Figure 2, only in the
as-grown samples, a shallow donor previously labelled E27
(Ref. 1) with an activation enthalpy of approximately
27 meV was observed. This donor has previously been attrib-
uted to the Zn
i
.
1,2
In the annealed samples, E27 could not be
detected and a shallow donor with an activation enthalpy of
35 meV was observed instead. There are two possibilities
explaining the absence of the E27 in the annealed samples:
(i) Either it was annealed-out as was also suggested by
Mtangi et al.,
2
or (ii) There is an increase in the acceptor
concentration with annealing, which in turn affects the
position of the Fermi level. In the latter case, the lowering of
the Fermi level would call for an increase in the degree of
acceptor compensation within the annealed material. E27
would then be ionized at all temperatures and, therefore, it
will not be observed by space charge spectroscopic techni-
ques. With the lowering of the Fermi level, its electrons in
the conduction band will easily be taken up by the acceptor
states. The failure to observe the E27 in the annealed sam-
ples is also in agreement with what was theoretically sug-
gested by Janotti and Van de Walle
21
and experimentally by
Neuvonen et al.
22
that the Zn
i
is highly mobile at tempera-
tures around 600
C and is not likely to be a stable donor.
The concentration of E35 is increased for the Ar and
vacuum annealed samples (Figure 2(a)). This increase in the
E35 concentration can be related to low oxygen partial pres-
sure. For the oxygen annealed samples, E35 has the highest
concentration in the sample annealed at 650
C. Annealing
at temperatures higher than 650
C resu lts in a decrease in
the low temperature capacitance (Figure 2(b)). This decrease
in capacitance witnessed for high temperature annealed
FIG. 1. Net doping density profiles of ZnO samples annealed in different
ambient conditions at 700
C. These profiles were recorded at room tempera-
ture and at a frequency of 50 kHz. Figure 1(b) Net doping density profiles of
ZnO samples annealed in oxygen ambient at different temperatures. These
profiles were recorded at room temperature and at a frequency of 50 kHz.
FIG. 2. Capacitance temperature scans for ZnO samples annealed in differ-
ent ambient conditions at a temperature of 700
C. These were recorded at a
reverse bias of 2.0 V scanning up in temperature. Figure 2(b) Capacitance
temperature scans for ZnO samples annealed in oxygen ambient at different
temperatures. These scans were recorded at a reverse bias of 2.0 V scanning
up in temperature. The inset shows the variation of capacitance with anneal-
ing temperature at 75 K.
124502-3 Mtangi et al. J. Appl. Phys. 113, 124502 (2013)
Downloaded 06 May 2013 to 137.215.6.53. This article is copyrighted as indicated in the abstract. Reuse of AIP content is subject to the terms at: http://jap.aip.org/about/rights_and_permissions

samples could be due to the increase in acceptor concentra-
tion with annealing temperature. The low temperature capac-
itance values show a pronounced trend wit h increase in
annealing temperature (inset of Figure 2(b)). The inset shows
the variation of capacitance with temperature at 75 K. The
capacitance increases with increase in annealing temperature
and reaches a maximum at an annealing temperature of
650
C, after which it decreases with increase in annealing
temperature.
B. Deep level defects
DLTS measurements performed on the un-annealed sam-
ple reveals the presence of five deep level defects (E1, E2,
E3, E4, and E5) (Figure 3). Arrhenius plots of these deep
level defects are presented in Figure 4. The activation enthal-
pies and apparent capture cross-sections of these defects are
presented in Tables I and II. The E4 and E5 deep levels are
being observed for the first time in the as-received samples
from Cermet. Since we have not observed the E4 and E5 in
as-received samples before,
13,18,19,23
we attribute these
defects to sample growth conditions. A defect with an activa-
tion enthalpy similar to the one we have measured has been
observed by Frank et al.
11
in vapour phase grown ZnO which
they attributed to an oxygen vacancy. Since our E4 defect
compares very well to the E4 by Frank et al.
11
in both activa-
tion enthalpy and capture cross-section, we can assign our E4
defect to the O-vacancy. Assignment of E4 to the oxygen
vacancy in this case implies that there is a high possibility
this particular batch of samples was grown under non-
equilibrium conditions, i.e., low oxygen partial pressures.
The activation enthalpy and apparent capture cross-section
of E5 have been obtained as 1.05 eV and 5 10
12
cm
2
,
respectively.
Interesting enough, annealing samples in different ambi-
ent conditions at 700
C anneal out the E4 and E5 deep level
defects. For samples annealed in oxygen ambient at different
temperatures (Figure 3(b)), below an annealin g temperature
of 675
C, E5 is annealed out, but E4 is still present. After
annealing at 675
C, both the E4 and E5 defects anneal-out.
Above the 800
C annealing temperature, the E2 defect
FIG. 3. Normalized DLTS spectra obtained from Pd/ZnO Schottky contacts
for ZnO samples annealed at 700
C in different ambient conditions. The
spectra were recorded at a rate window frequency of 100 Hz, reverse bias
voltage of 2.0 V, filling pulse of 0.3 V into forward bias and pulse width of
2.0 ms. Figure 3(b) Normalized DLTS spectra obtained from Pd/ZnO
Schottky contacts for ZnO samples annealed in oxygen ambient at different
temperatures. The spectra were recorded at a rate window frequency of 500
Hz, reverse bias voltage of 2.0 V, filling pulse of 0.4 V into forward bias and
pulse width of 2.0 ms. The inset shows the spectra obtained from the 900
C
annealed samples at a rate window frequency of 5 Hz.
FIG. 4. Arrhenius plots obtained from the ZnO samples annealed in different
ambient conditions at 700
C to calculate the activation enthalpy and appa-
rent capture cross-section of the observed defects. Figure 4(b) Arrhenius
plots obtained from the ZnO samples annealed at different temperatures in
oxygen ambient to calculate the activation enthalpy and apparent capture
cross-section of the observed defects.
124502-4 Mtangi et al. J. Appl. Phys. 113, 124502 (2013)
Downloaded 06 May 2013 to 137.215.6.53. This article is copyrighted as indicated in the abstract. Reuse of AIP content is subject to the terms at: http://jap.aip.org/about/rights_and_permissions