Acid mine drainage treatment by integrated submerged membrane distillation-sorption system.
TL;DR: The results showed that modified (heat treated) zeolite achieved 26-30% higher removal of heavy metals compared to natural untreatedZeolite, and the integrated system produced high quality fresh water while concentrating sulfuric acid and valuable heavy metals (Cu, Zn and Ni).
About: This article is published in Chemosphere.The article was published on 2019-03-01 and is currently open access. It has received 48 citations till now. The article focuses on the topics: Sorption & Membrane fouling.
Summary (2 min read)
Jump to: [2.2.2 Heat treated zeolite] – [2.3.1 Surface area and pore width distribution] – [2.3.3 Surface morphology and element contents] – [2.3.4 Influence of pH and surface charge] – [2.5.1 Membrane analysis] – [3.1 Performance of natural and modified (heat treated) zeolite] – [3.2.1 Permeate flux and quality] – [3.2.2. Membrane analysis] – [3.3 Performance of integrated submerged DCMD-sorption] – [3.3.1 Permeate flux and quality] and [3.3.2. Membrane analysis]
2.2.2 Heat treated zeolite
- Heat treatment method was used to potentially enhance the performance of natural zeolite (Motsi et al., 2009; Turner et al., 2000) .
- Heat treatment was chosen as it requires no additional chemicals and complex modification process.
- Heat treatment was carried out by placing an appropriate amount of powder form natural zeolite in a ceramic dish.
- The ceramic dish was then placed into preheated air atmosphere muffle furnace (Labec Laboratory Pty Ltd, NSW, Australia).
2.3.1 Surface area and pore width distribution
- Nitrogen adsorption test was used to determine the Brunauer-Emmett-Teller (BET) specific surface area and the Barrett-Joyner-Halenda (BJH) pore width distribution of the natural and heat treated zeolite samples.
- Nitrogen adsorption test was measured with a Micrometrics ASAP 2020 HD analyzer using low temperature, per the procedure of ISO 9277 and ISO 15901-2.
2.3.3 Surface morphology and element contents
- A scanning electron microscopy (SEM) ((Zeiss Supra 55VP Field Emission) was used to analyse the zeolite surface characteristics (before and upon sorption).
- The SEM was integrated with energy dispersive X-ray spectroscopy (EDX) (15kV accelerating voltage) in order to analyse the element contents in zeolite.
2.3.4 Influence of pH and surface charge
- Zeolite surface charge was determined using zeta potential measurement.
- For this purpose, zeolite (1 g/L) placed in beakers with 100ml AMD solution.
- The pH of the initial solutions were varied from 1 -9.
- Zetasizer (nano instrument ZS Zen3600, UK) was used to analyse the zeolite surface charge.
2.5.1 Membrane analysis
- The morphology and element composition on the surface of the used and virgin membranes were analysed using SEM-EDX at a voltage of 15 kV as per the details mentioned in Section 2.3.3.
- The hydrophobicity of the virgin and used membranes were evaluated by measuring the water contact angle of the membrane using a goniometer (Theta Lite, Biolin Scientific, Sweden).
- Measurements were duplicated at different location of the membrane and the average value was used for this study.
3.1 Performance of natural and modified (heat treated) zeolite
- The sorption capacity of natural and modified (heat treated) zeolite was tested for heavy metal removal from AMD.
- Higher heavy metal removal was achieved with heat treated zeolite compared to natural untreated zeolite (Table 3 ).
- Heating may have removed water on the surface as well as internal channels of the natural zeolite, resulting in vacant channels which enhances heavy metal sorption rate, as reported by previous studies (Ohgushi and Nagae, 2003; Turner et al., 2000) .
- Heavy metal removal by zeolite minimally improved beyond 500 °C of heating.
- This trend could be attributed to characteristics change of zeolite upon heat treatment.
3.2.1 Permeate flux and quality
- Meanwhile, the concentration of permeate solution remained low (TDS less than 0.01 g/L).
- The sulfate concentration in the permeate solution increased significantly from 0.13 mg/L to 50 mg/L.
3.2.2. Membrane analysis
- Visible brown deposition (resembling iron oxides) was observed on the used membrane (Fig. 8b ) compared to the virgin membrane (Fig. 8a ).
- SEM-EDX analysis revealed Fe, S and Al deposition on the membrane.
- The precipitated metals predominantly deposited on the membrane surface and was loosely attached to the surface.
- It is likely that the deposition only partially blocked the membrane pores, and therefore, a stable permeate flux was maintained throughout the operation duration.
- Nevertheless, the contact angle of the used membrane (68.6 ± 0.8°) reduced by 38 -40% compared to the virgin membrane (109.5 ± 0.5°), suggesting that the Fe deposition resulted in the reduction of membrane hydrophobicity and partial wetting of sulfate ions.
3.3 Performance of integrated submerged DCMD-sorption
- An integration of zeolite with submerged DCMD (Fig. 1 ) offers the potential for improving the performance of both processes in a single system.
- The integrated system enable zeolite to be used in fine powder form with long contact time (more than 24 h) when kept suspended in a storage tank.
- In return, the heavy metal removal by 500 °C heat treated zeolite (dose = 10.0 ± 0.2 g/L) at pH 4 will ensure minimal Fe and Al deposition onto the membrane during the submerged DCMD process.
3.3.1 Permeate flux and quality
- The integrated submerged DCMD-sorption system showed similar flux pattern as the submerged DCMD (Fig. 7 ), indicating that the DCMD performance was not affected by the presence of sorbent in the storage tank.
- The integrated system enabled to achieve high rejection of all ions, maintaining a permeate TDS of less than 0.01 g/L.
- The sulfate concentration in the feed solution was increased from 4.2 g/L to around 8.2 g/L, while the sulfate concentration in the permeate solution remained low (less than 0.13-0.15 mg/L).
3.3.2. Membrane analysis
- A simple heat treatment was effective to increase the performance of natural zeolite for heavy metal removal from AMD solution.
- Heat treatment of natural zeolite at 500 °C enhanced heavy metal removal by 26-30%.
Did you find this useful? Give us your feedback
Figures (15)

Fig. 6. Influence of pH on heavy metal removal from AMD solution with 500 °C heated 425 zeolite (dose = 10 ± 0.2 g/L) 426 
Table 3 Performance of natural and heat treated zeolite with AMD solution (sorbent dose 5.0 280 ± 0.2 g/L, pH 2.0 ± 0.2). 281 
Table 7 Pseudo first and second order kinetic parameters for heavy metal sorption from 361 AMD solution at pH 2.0 ± 0.2 using 500 °C heat treated zeolite (dose =10 ± 0.2 g/L) 362 
Table 1 Characteristics of synthetic AMD 131 
Fig. 8. SEM-EDX of hollowfiber membrane (a) virgin (b) used membrane with AMD in 483 submerged DCMD (c) used membrane with AMD in integrated DCMD-sorption. 484 
Fig. 1. Set-up of integrated submerged DCMD – sorption system 259 2.5.1 Membrane analysis 260 The morphology and element composition on the surface of the used and virgin membranes 261 were analysed using SEM-EDX at a voltage of 15 kV as per the details mentioned in Section 262 2.3.3. The hydrophobicity of the virgin and used membranes were evaluated by measuring 263 the water contact angle of the membrane using a goniometer (Theta Lite, Biolin Scientific, 264 Sweden). Measurements were duplicated at different location of the membrane and the 265 average value was used for this study. 266 
Fig. 4. EDX results of natural and 500 °C heat treated zeolite (before and after heavy metal 321 sorption). 322 323 324 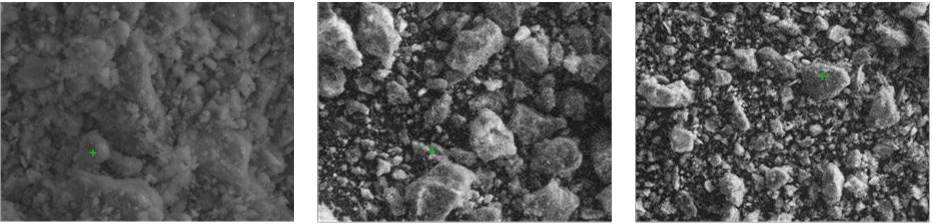
Fig. 3. SEM images of (a) natural untreated zeolite and 500 °C heat treated zeolite (b) 318 unused/before sorption and (c) after heavy metal sorption 319 
Fig. 7. Permeate flux for AMD treatment using submerged DCMD and integrated submerged 480 DCMD –sorption system 481 482 
Table 2 Natural zeolite chemical contents 151 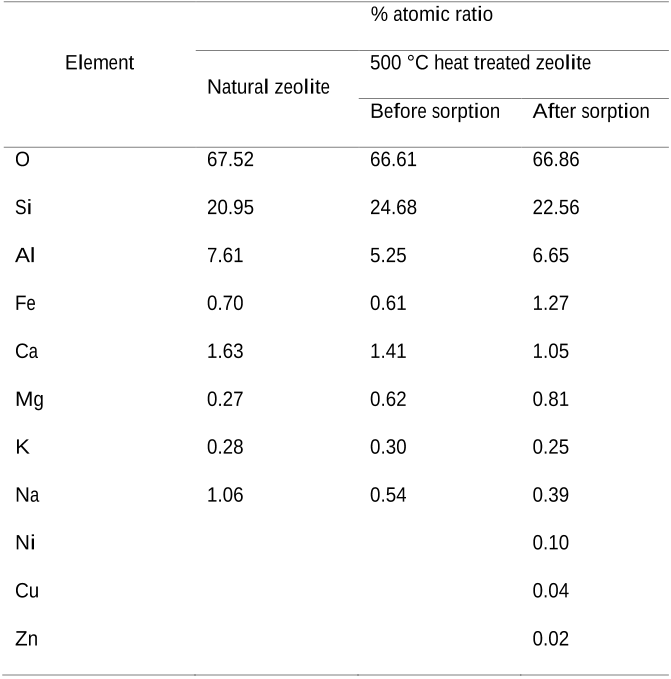
Table 5 EDX results of natural and 500 °C heat treated zeolite 325 
Fig. 2. XRD peaks of natural and heat treated zeolite 306 307 
Fig. 5. 500 °C heat treated zeolite (a) heavy metal removal as a function of sorbent dose (pH 416 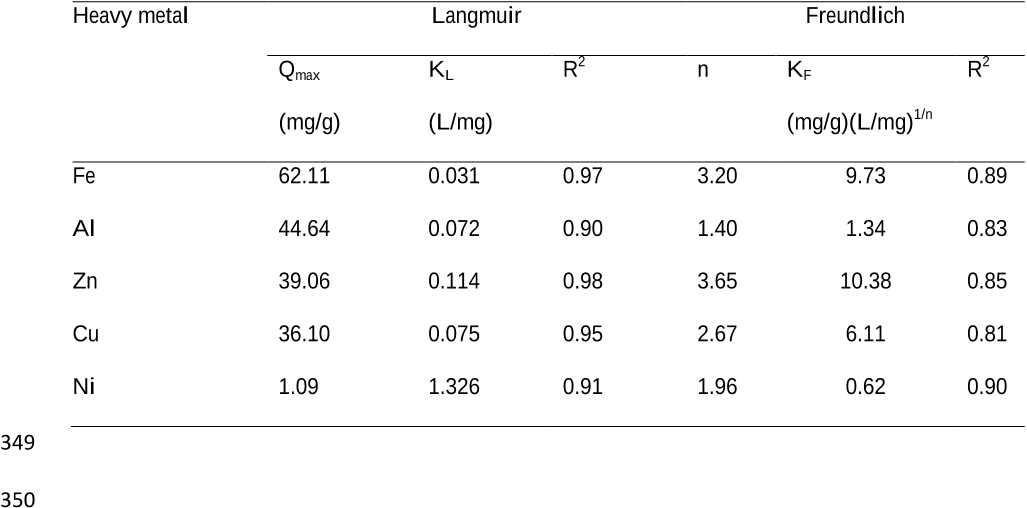
Table 6 Isotherm data (Langmuir and Freundlich) for heavy metal sorption from AMD 347 solution at pH 2.0 ± 0.2 using 500 °C heat treated zeolite. 348 
Table 4 Surface area and pore width of natural and heat treated zeolite. 297
Citations
More filters
TL;DR: The mining and hydrometallurgical industries generate effluents characterised by a high acidity (pH) as mentioned in this paper, which is the acidity of the effluent.
48 citations
TL;DR: In this paper, a review describes the mechanism of formation and the effects of acid mine drainage (AMD), presents the prevention and treatment technologies, identifies critical research gaps, and explores the challenges and opportunities encountered by AMD.
42 citations
TL;DR: In this paper, direct contact membrane distillation (DCMD) has been used for acid mine drainage and pickling solutions, which is a result of certain sulfide minerals oxidation.
41 citations
TL;DR: In this paper, the authors focus on new opportunities for metal resource recovery from seawater desalination brine, while outlining associated sustainability challenges and opportunities, and discuss the potential of metals recovery.
Abstract: The urgent need for environmental sustainability has increasingly prompted policy makers to emphasize resource recovery from desalination brine streams. Recent research on resource recovery from waste streams has shown rising momentum with near term viability for several new technologies. In this perspective, we focus on new opportunities for metal resource recovery from seawater desalination brine, while outlining associated sustainability challenges and opportunities. The potential of metals recovery is discussed.
38 citations
TL;DR: In this article, mesoporous silica particles, based on SBA-15 matrix, were functionalized with triethylenetetramine (TETA), and characterized by transmission electron microscopy (TEM), small angle X-ray scattering (SAXS), and N2-adsorption/desorption isotherms (surface area and pore size distribution).
Abstract: Mesoporous silica particles, based on SBA-15 matrix, were functionalized with triethylenetetramine (TETA), and characterized by transmission electron microscopy (TEM), small angle X-ray scattering (SAXS), and N2-adsorption/desorption isotherms (surface area and pore size distribution). The functionalization of SBA-15 with TETA to obtain SBA-TETA was confirmed by Fourier transform infrared spectroscopy (FTIR) and thermogravimetric analysis (TGA). SBA-TETA functional material was used for the adsorption of Cu2+ and Zn2+ metal ions from aqueous solutions at pH 4 and T = 298 K. The adsorption kinetics was faster for Zn2+ with respect to Cu2+ ions, and could be described by the pseudo-second order model indicating the chemisorption of the metal ions as the rate-determining step of the adsorption process. Adsorption isotherms at 298 K were carried out, and the experimental data analyzed with Freundlich, Temkin, and Langmuir models. Among the isotherm models, Langmuir gave the best fitting of the experimental data, allowing to quantify the maximal adsorbable amount of Cu2+ (23.9 mg g−1) and Zn2+ (13.6 mg g−1) by SBA-TETA. Moreover, the Langmuir constant, KL, was used to calculate the thermodynamic adsorption constant Ko and the associated ΔGo, namely –21.7 kJ mol−1 and –28.4 kJ mol−1 for Cu2+ and Zn2+ adsorption processes, respectively. These values are better than those reported in similar works, likely due to the superior performance of TETA chelating agent respect to conventional alkyl-amino ligands once grafted on SBA-15 surface.
36 citations
References
More filters
01 Dec 2006
TL;DR: The study was carried out on the sorption of heavy metals under static conditions from single- and multicomponent aqueous solutions by raw and pretreated clinoptilolite and results fit well to the Langmuir and the Freundlich models.
Abstract: The study was carried out on the sorption of heavy metals (Ni2+, Cu2+, Pb2+, and Cd2+) under static conditions from single- and multicomponent aqueous solutions by raw and pretreated clinoptilolite. The sorption has an ion-exchange nature and consists of three stages, i.e., the adsorption on the surface of microcrystals, the inversion stage, and the moderate adsorption in the interior of the microcrystal. The finer clinoptilolite fractions sorb higher amounts of the metals due to relative enriching by the zeolite proper and higher cleavage. The slight difference between adsorption capacity of the clinoptilolite toward lead, copper, and cadmium from single- and multicomponent solutions may testify to individual sorption centers of the zeolite for each metal. The decrease of nickel adsorption from multicomponent solutions is probably caused by the propinquity of its sorption forms to the other metals and by competition. The maximum sorption capacity toward Cd2+ is determined as 4.22 mg/g at an initial concentration of 80 mg/L and toward Pb2+, Cu2+, and Ni2+ as 27.7, 25.76, and 13.03 mg/g at 800 mg/L. The sorption results fit well to the Langmuir and the Freundlich models. The second one is better for adsorption modeling at high metal concentrations.
546 citations
TL;DR: In this article, the adsorption behavior of natural zeolite (clinoptilolite) has been studied in order to determine its applicability in treating acid mine drainage.
499 citations
TL;DR: This mineral showed the same high sorption capacity values when used in the purification of metal electroplating waste waters, appearing, therefore, as most suitable to perform metal waste water purification processes.
482 citations
TL;DR: The immobilization of the metals during pH increase and the subsequent remobilization caused by re-acidification can be well described by a geochemical equilibrium speciation model that accounts for metal complexation at hydrous ferric oxides, for ion exchange on the zeolite surfaces, as well as for dissolution and precipitation processes.
Abstract: In this study, we investigated the removal of Fe, Pb, Cd, and Zn from synthetic mine waters by a natural zeolite. The emphasis was given to the zeolite's behavior toward a few cations in competition with each other. Pb was removed efficiently from neutral as well as from acidic solutions, whereas the uptake of Zn and Cd decreased with low pH and high iron concentrations. With increasing Ca concentrations in solution, elimination of Zn and Cd became poorer while removal of Pb remained virtually unchanged. The zeolite was stable in acidic solutions. Disintegration was only observed below pH 2.0. Forward- and back-titration of synthetic acidic mine water were carried out in the presence and absence of zeolite to simulate the effects of a pH increase by addition of neutralizing agents and a re-acidification which can be caused by subsequent mixing with acidic water. The pH increase during neutralization causes precipitation of hydrous ferric oxides and decreased dissolved metal concentrations. Zeolite addition further diminished Pb concentrations but did not have an effect on Zn and Cd concentrations in solution. During re-acidification of the solution, remobilization of Pb was weaker in the presence than in the absence of zeolite. No substantial differences were observed for Fe, Cd, and Zn immobilization. The immobilization of the metals during pH increase and the subsequent remobilization caused by re-acidification can be well described by a geochemical equilibrium speciation model that accounts for metal complexation at hydrous ferric oxides, for ion exchange on the zeolite surfaces, as well as for dissolution and precipitation processes.
346 citations
TL;DR: In this paper, a cancrinite-type zeolite (ZFA) was synthesized from Class C fly ash via the molten-salt method and the maximum exchange level (MEL) was found that the uptake of heavy metals on ZFA was subjected to an ion exchange mechanism.
271 citations