Acid mine drainage treatment by integrated submerged membrane distillation-sorption system.
TL;DR: The results showed that modified (heat treated) zeolite achieved 26-30% higher removal of heavy metals compared to natural untreatedZeolite, and the integrated system produced high quality fresh water while concentrating sulfuric acid and valuable heavy metals (Cu, Zn and Ni).
About: This article is published in Chemosphere.The article was published on 2019-03-01 and is currently open access. It has received 48 citations till now. The article focuses on the topics: Sorption & Membrane fouling.
Summary (2 min read)
Jump to: [2.2.2 Heat treated zeolite] – [2.3.1 Surface area and pore width distribution] – [2.3.3 Surface morphology and element contents] – [2.3.4 Influence of pH and surface charge] – [2.5.1 Membrane analysis] – [3.1 Performance of natural and modified (heat treated) zeolite] – [3.2.1 Permeate flux and quality] – [3.2.2. Membrane analysis] – [3.3 Performance of integrated submerged DCMD-sorption] – [3.3.1 Permeate flux and quality] and [3.3.2. Membrane analysis]
2.2.2 Heat treated zeolite
- Heat treatment method was used to potentially enhance the performance of natural zeolite (Motsi et al., 2009; Turner et al., 2000) .
- Heat treatment was chosen as it requires no additional chemicals and complex modification process.
- Heat treatment was carried out by placing an appropriate amount of powder form natural zeolite in a ceramic dish.
- The ceramic dish was then placed into preheated air atmosphere muffle furnace (Labec Laboratory Pty Ltd, NSW, Australia).
2.3.1 Surface area and pore width distribution
- Nitrogen adsorption test was used to determine the Brunauer-Emmett-Teller (BET) specific surface area and the Barrett-Joyner-Halenda (BJH) pore width distribution of the natural and heat treated zeolite samples.
- Nitrogen adsorption test was measured with a Micrometrics ASAP 2020 HD analyzer using low temperature, per the procedure of ISO 9277 and ISO 15901-2.
2.3.3 Surface morphology and element contents
- A scanning electron microscopy (SEM) ((Zeiss Supra 55VP Field Emission) was used to analyse the zeolite surface characteristics (before and upon sorption).
- The SEM was integrated with energy dispersive X-ray spectroscopy (EDX) (15kV accelerating voltage) in order to analyse the element contents in zeolite.
2.3.4 Influence of pH and surface charge
- Zeolite surface charge was determined using zeta potential measurement.
- For this purpose, zeolite (1 g/L) placed in beakers with 100ml AMD solution.
- The pH of the initial solutions were varied from 1 -9.
- Zetasizer (nano instrument ZS Zen3600, UK) was used to analyse the zeolite surface charge.
2.5.1 Membrane analysis
- The morphology and element composition on the surface of the used and virgin membranes were analysed using SEM-EDX at a voltage of 15 kV as per the details mentioned in Section 2.3.3.
- The hydrophobicity of the virgin and used membranes were evaluated by measuring the water contact angle of the membrane using a goniometer (Theta Lite, Biolin Scientific, Sweden).
- Measurements were duplicated at different location of the membrane and the average value was used for this study.
3.1 Performance of natural and modified (heat treated) zeolite
- The sorption capacity of natural and modified (heat treated) zeolite was tested for heavy metal removal from AMD.
- Higher heavy metal removal was achieved with heat treated zeolite compared to natural untreated zeolite (Table 3 ).
- Heating may have removed water on the surface as well as internal channels of the natural zeolite, resulting in vacant channels which enhances heavy metal sorption rate, as reported by previous studies (Ohgushi and Nagae, 2003; Turner et al., 2000) .
- Heavy metal removal by zeolite minimally improved beyond 500 °C of heating.
- This trend could be attributed to characteristics change of zeolite upon heat treatment.
3.2.1 Permeate flux and quality
- Meanwhile, the concentration of permeate solution remained low (TDS less than 0.01 g/L).
- The sulfate concentration in the permeate solution increased significantly from 0.13 mg/L to 50 mg/L.
3.2.2. Membrane analysis
- Visible brown deposition (resembling iron oxides) was observed on the used membrane (Fig. 8b ) compared to the virgin membrane (Fig. 8a ).
- SEM-EDX analysis revealed Fe, S and Al deposition on the membrane.
- The precipitated metals predominantly deposited on the membrane surface and was loosely attached to the surface.
- It is likely that the deposition only partially blocked the membrane pores, and therefore, a stable permeate flux was maintained throughout the operation duration.
- Nevertheless, the contact angle of the used membrane (68.6 ± 0.8°) reduced by 38 -40% compared to the virgin membrane (109.5 ± 0.5°), suggesting that the Fe deposition resulted in the reduction of membrane hydrophobicity and partial wetting of sulfate ions.
3.3 Performance of integrated submerged DCMD-sorption
- An integration of zeolite with submerged DCMD (Fig. 1 ) offers the potential for improving the performance of both processes in a single system.
- The integrated system enable zeolite to be used in fine powder form with long contact time (more than 24 h) when kept suspended in a storage tank.
- In return, the heavy metal removal by 500 °C heat treated zeolite (dose = 10.0 ± 0.2 g/L) at pH 4 will ensure minimal Fe and Al deposition onto the membrane during the submerged DCMD process.
3.3.1 Permeate flux and quality
- The integrated submerged DCMD-sorption system showed similar flux pattern as the submerged DCMD (Fig. 7 ), indicating that the DCMD performance was not affected by the presence of sorbent in the storage tank.
- The integrated system enabled to achieve high rejection of all ions, maintaining a permeate TDS of less than 0.01 g/L.
- The sulfate concentration in the feed solution was increased from 4.2 g/L to around 8.2 g/L, while the sulfate concentration in the permeate solution remained low (less than 0.13-0.15 mg/L).
3.3.2. Membrane analysis
- A simple heat treatment was effective to increase the performance of natural zeolite for heavy metal removal from AMD solution.
- Heat treatment of natural zeolite at 500 °C enhanced heavy metal removal by 26-30%.
Did you find this useful? Give us your feedback
Figures (15)

Fig. 6. Influence of pH on heavy metal removal from AMD solution with 500 °C heated 425 zeolite (dose = 10 ± 0.2 g/L) 426 
Table 3 Performance of natural and heat treated zeolite with AMD solution (sorbent dose 5.0 280 ± 0.2 g/L, pH 2.0 ± 0.2). 281 
Table 7 Pseudo first and second order kinetic parameters for heavy metal sorption from 361 AMD solution at pH 2.0 ± 0.2 using 500 °C heat treated zeolite (dose =10 ± 0.2 g/L) 362 
Table 1 Characteristics of synthetic AMD 131 
Fig. 8. SEM-EDX of hollowfiber membrane (a) virgin (b) used membrane with AMD in 483 submerged DCMD (c) used membrane with AMD in integrated DCMD-sorption. 484 
Fig. 1. Set-up of integrated submerged DCMD – sorption system 259 2.5.1 Membrane analysis 260 The morphology and element composition on the surface of the used and virgin membranes 261 were analysed using SEM-EDX at a voltage of 15 kV as per the details mentioned in Section 262 2.3.3. The hydrophobicity of the virgin and used membranes were evaluated by measuring 263 the water contact angle of the membrane using a goniometer (Theta Lite, Biolin Scientific, 264 Sweden). Measurements were duplicated at different location of the membrane and the 265 average value was used for this study. 266 
Fig. 4. EDX results of natural and 500 °C heat treated zeolite (before and after heavy metal 321 sorption). 322 323 324 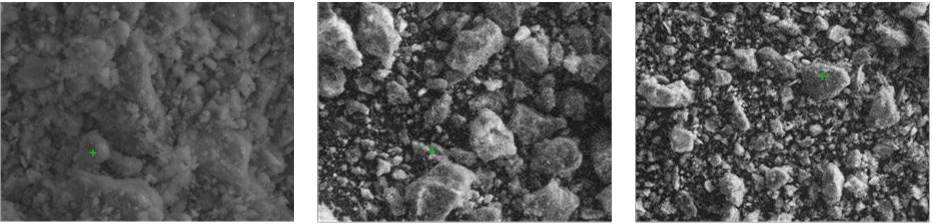
Fig. 3. SEM images of (a) natural untreated zeolite and 500 °C heat treated zeolite (b) 318 unused/before sorption and (c) after heavy metal sorption 319 
Fig. 7. Permeate flux for AMD treatment using submerged DCMD and integrated submerged 480 DCMD –sorption system 481 482 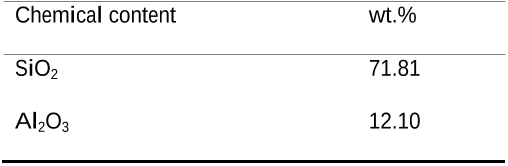
Table 2 Natural zeolite chemical contents 151 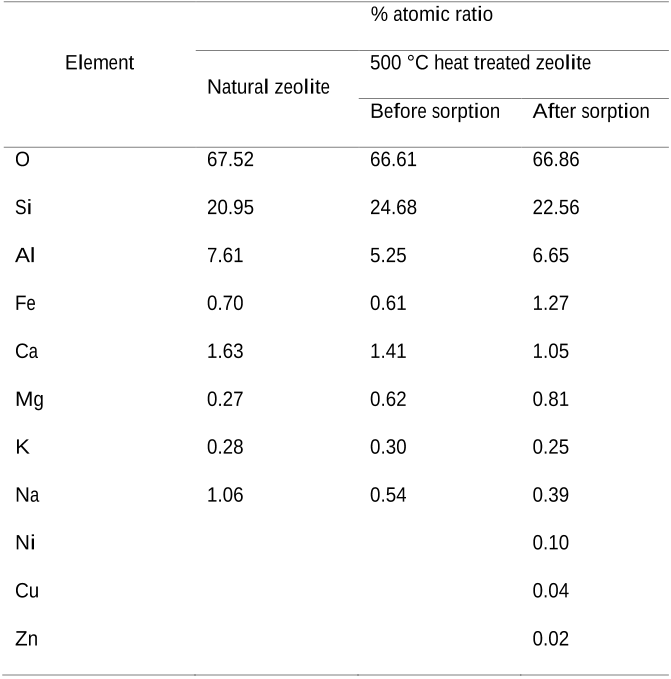
Table 5 EDX results of natural and 500 °C heat treated zeolite 325 
Fig. 2. XRD peaks of natural and heat treated zeolite 306 307 
Fig. 5. 500 °C heat treated zeolite (a) heavy metal removal as a function of sorbent dose (pH 416 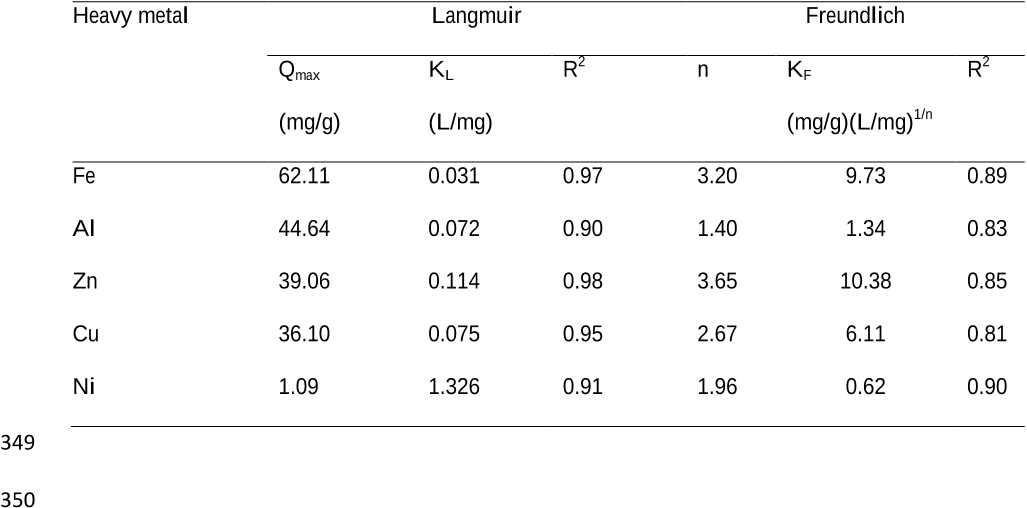
Table 6 Isotherm data (Langmuir and Freundlich) for heavy metal sorption from AMD 347 solution at pH 2.0 ± 0.2 using 500 °C heat treated zeolite. 348 
Table 4 Surface area and pore width of natural and heat treated zeolite. 297
Citations
More filters
TL;DR: Insight is provided in establishing reuse and resource recovery as the holistic approach towards sustainable AMD treatment and integrated technologies that deserve in depth future exploration are highlighted.
229 citations
TL;DR: In this article, the performance and related challenges of various hybrid Membrane Distillation (MD) systems with a focus on resource recovery are discussed. And future suggestions for improving hybrid MD are discussed, specifically pilot-scale application, module configuration and membrane development.
85 citations
TL;DR: In this article, the authors review several treatment options such as selective metal precipitation, adsorption, electrochemical processes and membrane processes for acid mine drainage, and conclude that membrane processes are the most promising according to lab-scale results, notably because high quality water is obtained.
Abstract: Acid mine drainage induced by the mining industry causes environmental and economic issues. Acid mine drainage contains mainly metals such as Fe, Al, Cu, Ca, Mg, Mn and Zn. Preventing the formation of acid mine drainage has not been found feasible. As a consequence, remediation treatments have been developed during the last years to remove metals and obtain high-quality water, which may be reused. We review here several treatment options such as selective metal precipitation, adsorption, electrochemical processes and membrane processes. Adsorption is the most employed commercially since it can recover 99% of the metals. Membrane processes are promising according to lab-scale results, notably because high-quality water is obtained. Further research is necessary to implement combination of technologies, e.g., adsorption membrane, at larger scales, as well as to obtain more valuable products that can balance the overall economy for the mining industry.
71 citations
TL;DR: This study proposes three more sustainable solutions for reducing and eventually eliminating the impact of AMD with less capital investment while also resolving the landfill problem as well.
57 citations
TL;DR: Excellent stability of P-60S-EDTA membrane in continuous operation of 36 h in both ideal and practical water environment suggests its promising application in real field heavy metal contaminated waste water treatment.
51 citations
References
More filters
TL;DR: The clinoptilolite rich zeolite from Bigadic which was formed from alteration of volcanic glass were treated with acidic (HCl, HBO3, H3PO4), alkaline (KOH, NaOH) solutions as mentioned in this paper.
Abstract: The clinoptilolite rich zeolite from Bigadic which was formed from alteration of volcanic glass were treated with acidic (HCl, H3BO3, H3PO4), alkaline (KOH, NaOH) solutions. Hydrothermally treated and untreated samples were heat treated at 400, 550 and 700°C. XRD, ICP-MS and N2 gas adsorption were used for physicochemical characterization of zeolites. Considering the Si/Al > 4 and Na+K/Ca+Mg H3PO4 > HBO3 > KOH > NaOH. XRD analysis indicated that the structure of zeolite was not altered with acids and alkali treatments. The structure of zeolite treated with HCl and other acids started to deform at 400 and 550°C respectively. In treatment with HCl, Si/Al ratio increases with significant a decrease in K content which resulted in a decrease in the heat stability of zeolite. No change was observed in the structure and thermal stability of clinoptilolite after alkali treatments. The fact that although significant amount of Na is removed with H3BO3 acid and Na is increased with NaOH but the thermal stability remains the same indicates that Na cation is not an important parameter as much as K. HCl and H3PO4 acid treatments increased the surface area depending on the dissolution of amorphous material and H3PO4 was found to be more effective. However, the total pore size decreased due to formation of new micropores.
18 citations
TL;DR: NZVI-DE is demonstrated as highly selective for Cu removal with >99% uptake recorded after 0.25 h, and holds great promise as a novel 'Precision Mining' process for the rapid and selective Cu recovery from acidic wastewater, process effluents and leach liquors.
17 citations
Book Chapter•
01 Jan 2012
TL;DR: In this paper, a low-energy direct contact membrane distillation (CDMD) was used for the recovery of water and acid from acidic waste solutions generated in the mining industry.
Abstract: This paper describes initial laboratory results from the novel application of the low-energy direct contact membrane distillation (CDMD) process for the recovery of water and acid from acidic waste solutions generated in the mining industry.
10 citations
TL;DR: Given in situ sediment metal contamination is very localised, it appears on a river reach scale that the acid drainage precipitates will not significantly contribute, over and above, the background release of these metals during these conditions.
9 citations