
Eur.
J.
Biochem.
105,
275-278 (1980)
Addition of Glucose to Dolichyl Diphosphate Oligosaccharide
and Transfer to Protein
Roberto
J.
STANELONI, Rodolfo A. UGALDE, and Luis F. LELOIR
Instituto de Investigaciones Bioquimicas “Fundacion Campomar” and Facultad de Ciencias Exactas
y
Naturales, Buenos Aires
(Received August
31,
1979)
The glycosylation of asparagine residues in proteins is known to occur by transfer from a dolichyl
diphosphate oligosaccharide containing glucose. Paper chromatography allowed the separation of
oligosaccharides (obtained by acid hydrolysis of the dolichyl diphosphate derivative) containing 1,
2 and
3
glucose residues. Using this procedure it was found that the addition of all three glucoses
to the dolichyl diphosphate oligosaccharide occur with dolichyl phosphate glucose as donor. Further-
more only the compound with three glucoses was used as donor in the transfer
to
protein. The
addition of glucose to exogenous dolichyldiphosphate oligosaccharide labelled by transfer from radio-
active guanosine diphosphate mannose was detected.
There is evidence showing that the donor for the
glycosylation of asparagine residues in proteins is a
dolichyl diphosphate oligosaccharide
[l
-
21. The evi-
dence shows that this oligosaccharide, whose structure
is known [3], is built up by the transfer of acetyl-
glucosamine phosphate to dolichyl phosphate fol-
lowed by the addition of one more acetylglucosamine,
nine mannoses and one to three glucoses. The oligo-
saccharide is then transferred to protein and the
glucose residues are removed. The complex oligo-
saccharides may then be formed by removal of six
mannoses and addition of acetylglucosamine, galac-
tose and sialic acid. Another pathway consisting in
removal or addition
of
mannose residues would lead
to the formation of the high-mannose-type oligo-
saccharides.
This paper reports studies on some aspects of the
process of glucose addition to the oligosaccharide and
of transfer
of
the latter to protein.
MATERIALS AND METHODS
Rat liver microsomes were prepared as described
previously [4]. Dol-P was isolated and purified up to
the DEAE-cellulose step [5]. Do~-P-[~~C]G~C was
prepared by incubation of UDP-[14C]Glc with liver
microsomes and purified by DEAE-cellulose chro-
matography [5,6].
Dol-P-P-Glc-oligosaccharide
la-
belled in the glucose or in the mannose was obtained
by incubation of microsomes with UDP-[’4C]G1~ [7]
Abbreviations.
The glucose-containing oligosaccharides with
three, two and one glucose residues are referred
to
as Glc3, Glcz
and Glcl oligosaccharides.
Dol,
dolichyl; Glc, glucose; Man,
mannose;
P,
phosphate.
or GDP-[’4C]Man [8] respectively. Dol-P-P-Glc-oligo-
saccharide labelled in mannose and glucose was ob-
tained from oviduct slices incubated with [‘4C]-man-
nose [9,10]. Free oligosaccharides were obtained from
the dolichyl diphosphate derivatives by mild acid
hydrolysis [lo].
Paper chromatography of the oligosaccharides was
carried out with the following solvent: 1-propanol/
nitromethane/water
(5
:
2
:
4). This mixture is referred
to as the propanol-nitromethane solvent.
RESULTS
Preparation
of
Dolichyl-Diphosphate-Glucose-Con-
taining Oligosaccharides. Incubation
of
UDP-[14C]G1~
with liver microsomes leads to the formation of la-
belled Dol-P-P oligosaccharides. Mild acid hydrolysis
of these compounds results in the liberation of free
oligosaccharides which can be separated by paper
chromatography into three different compounds. This
separation was first obtained by Kornfeld et al. [ll]
with
3
-butanol/pyridine/water (4:
3
:
4) as solvent in
a 16-day run. With the propanol/nitromethane solvent
the separation can
be
obtained in
7
days (Fig. 1).
The relative amounts of the different glucose-
containing oligosaccharides vary with the conditions
used for the synthesis. The changes at various incuba-
tion times are shown in Fig.
2.
The Glc3-oligosaccharide
increases up to 20min and then decreases progressively.
The Glc2-oligosaccharide follows a similar course but
does not decrease as fast while Glcl-oligosaccharide
remains constant. When Glcl and Glc2 oligosacchari-
des had to be prepared an incubation time of
80
min
was used. Under these conditions separation by paper
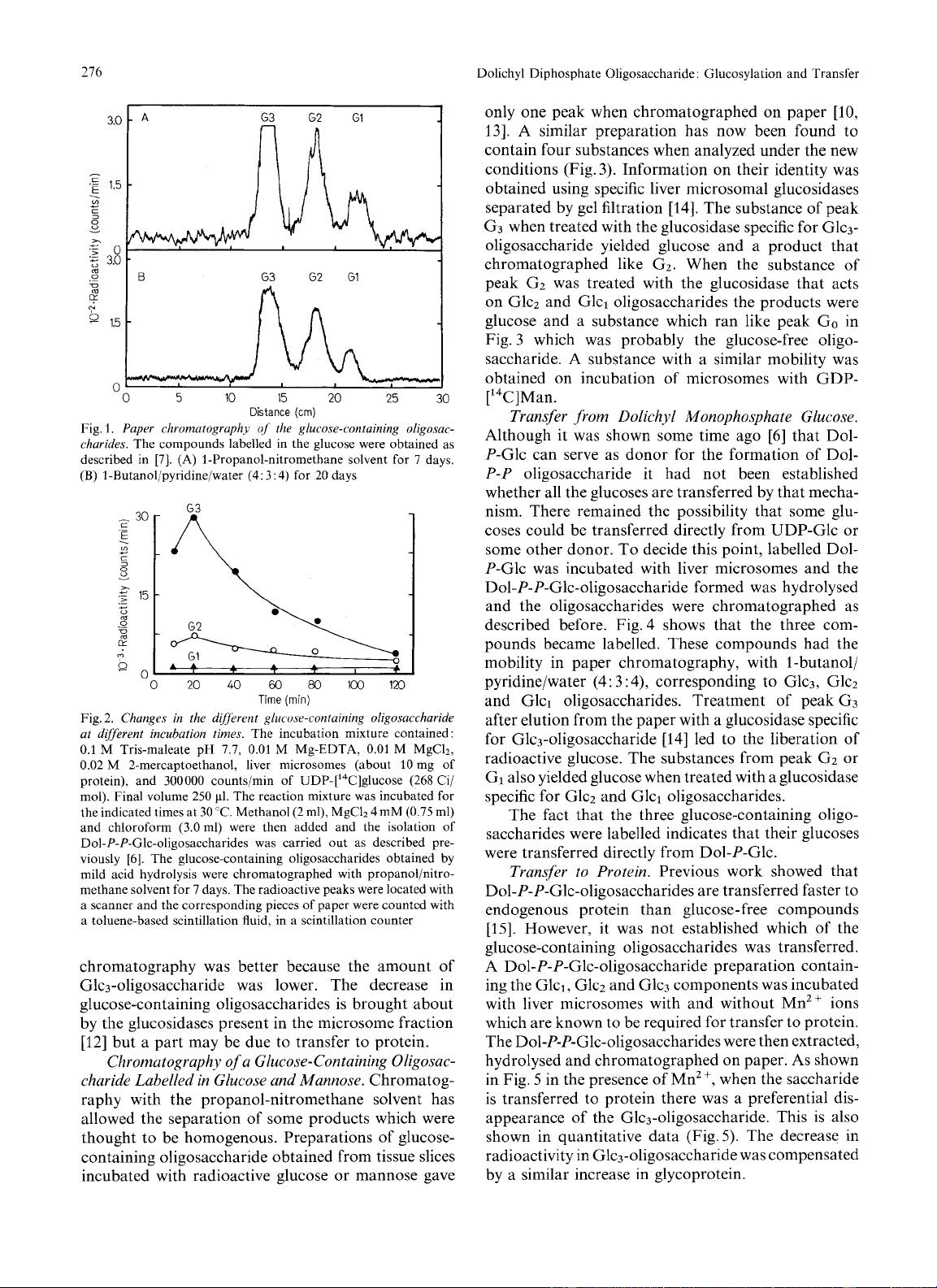
216
Dolichyl Diphosphate Oligosaccharide: Glucosylation and Transfer
.~
2
I0
G3 G2
G1
I
0
5
10
15 20 25
30
Distance
(cm)
Fig.
1.
Paper chromatogruphy
qJ'
the glucose-containing oligosac-
charides.
The compounds labelled in the glucose were obtained as
described in
[7].
(A) 1-Propanol-nitromethane solvent for
7
days,
(B)
I-Butanol/pyridine/water
(4:
3:
4)
for 20 days
Fig. 2.
Changes
in
the
dij'irent
glucose-conluining oligosaccharide
at different incubation times.
The incubation mixture contained
:
0.1
M Tris-maleate pH
7.7,
0.01
M
Mg-EDTA,
0.01
M MgC12,
0.02 M 2-mercaptoethanol, liver microsomes (about 10 mg of
protein), and 300000 counts/min of UDP-['4C]glucose (268 Ci/
mol). Final volume
250
PI.
The reaction mixture was incubated for
the indicated times at 30 "C. Methanol (2 ml), MgCh
4mM
(0.75
ml)
and chloroform (3.0ml) were then added and the isolation
of
Dol-P-P-Glc-oligosaccharides
was carried out as described pre-
viously [6]. The glucose-containing oligosaccharides obtained by
mild acid hydrolysis were chromatographed with propanol/nitro-
methane solvent for
7
days. The radioactive peaks were located with
a scanner and the corresponding pieces
of
paper were counted with
a toluene-based scintillation fluid, in a scintillation counter
chromatography was better because the amount of
Glc3-oligosaccharide was lower. The decrease in
glucose-containing oligosaccharides is brought about
by the glucosidases present in the microsome fraction
[I21
but a part may be due to transfer to protein.
Chromatography
of
a Glucose-Containing Oligosac-
charide Labelled
in
Glucose and Mannose.
Chromatog-
raphy with the propanol-nitromethane solvent has
allowed the separation of some products which were
thought to be homogenous. Preparations of glucose-
containing oligosaccharide obtained from tissue slices
incubated with radioactive glucose or mannose gave
only one peak when chromatographed on paper [lo,
131.
A
similar preparation has now been found to
contain four substances when analyzed under the new
conditions (Fig. 3). Information on their identity was
obtained using specific liver microsomal glucosidases
separated by gel filtration
[14].
The substance of peak
G3 when treated with the glucosidase specific for Gk3-
oligosaccharide yielded glucose and a product that
chromatographed like G2. When the substance of
peak G2 was treated with the glucosidase that acts
on Glc2 and Glcl oligosaccharides the products were
glucose and a substance which ran like peak
Go
in
Fig. 3 which was probably the glucose-free oligo-
saccharide.
A
substance with a similar mobility was
obtained on incubation of microsomes with GDP-
['4C]Man.
Transfer from Dolichyl Monophosphate Glucose.
Although it was shown some time ago
[6]
that Dol-
P-Glc can serve as donor for the formation of Dol-
P-P oligosaccharide it had not been established
whether all the glucoses are transferred by that mecha-
nism. There remained the possibility that some glu-
coses could be transferred directly from UDP-Glc or
some other donor. To decide this point, labelled Dol-
P-Glc was incubated with liver microsomes and the
Dol-P-P-Glc-oligosaccharide
formed was hydrolysed
and the oligosaccharides were chromatographed as
described before. Fig.4 shows that the three com-
pounds became labelled. These compounds had the
mobility in paper chromatography, with 1-butanol/
pyridine/water (4
:
3
:
4),
corresponding to Glc3, Glc2
and Glcl oligosaccharides. Treatment of peak G3
after elution from the paper with a glucosidase specific
for Glc3-oligosaccharide [14] led to the liberation of
radioactive glucose. The substances from peak GZ or
G1 also yielded glucose when treated with a glucosidase
specific for Glc2 and Glcl oligosaccharides.
The fact that the three glucose-containing oligo-
saccharides were labelled indicates that their glucoses
were transferred directly from Dol-P-Glc.
Transfer to Protein.
Previous work showed that
Dol-P-P-Glc-oligosaccharides
are transferred faster to
endogenous protein than glucose-free compounds
[15].
However, it was not established which of the
glucose-containing oligosaccharides was transferred.
A
Dol-P-P-Glc-oligosaccharide
preparation contain-
ing the Glcl, Glc2 and Glc3 components was incubated
with liver microsomes with and without Mn2+ ions
which are known to be required for transfer to protein.
The Dol-P-P-Glc-oligosaccharides were then extracted,
hydrolysed and chromatographed on paper.
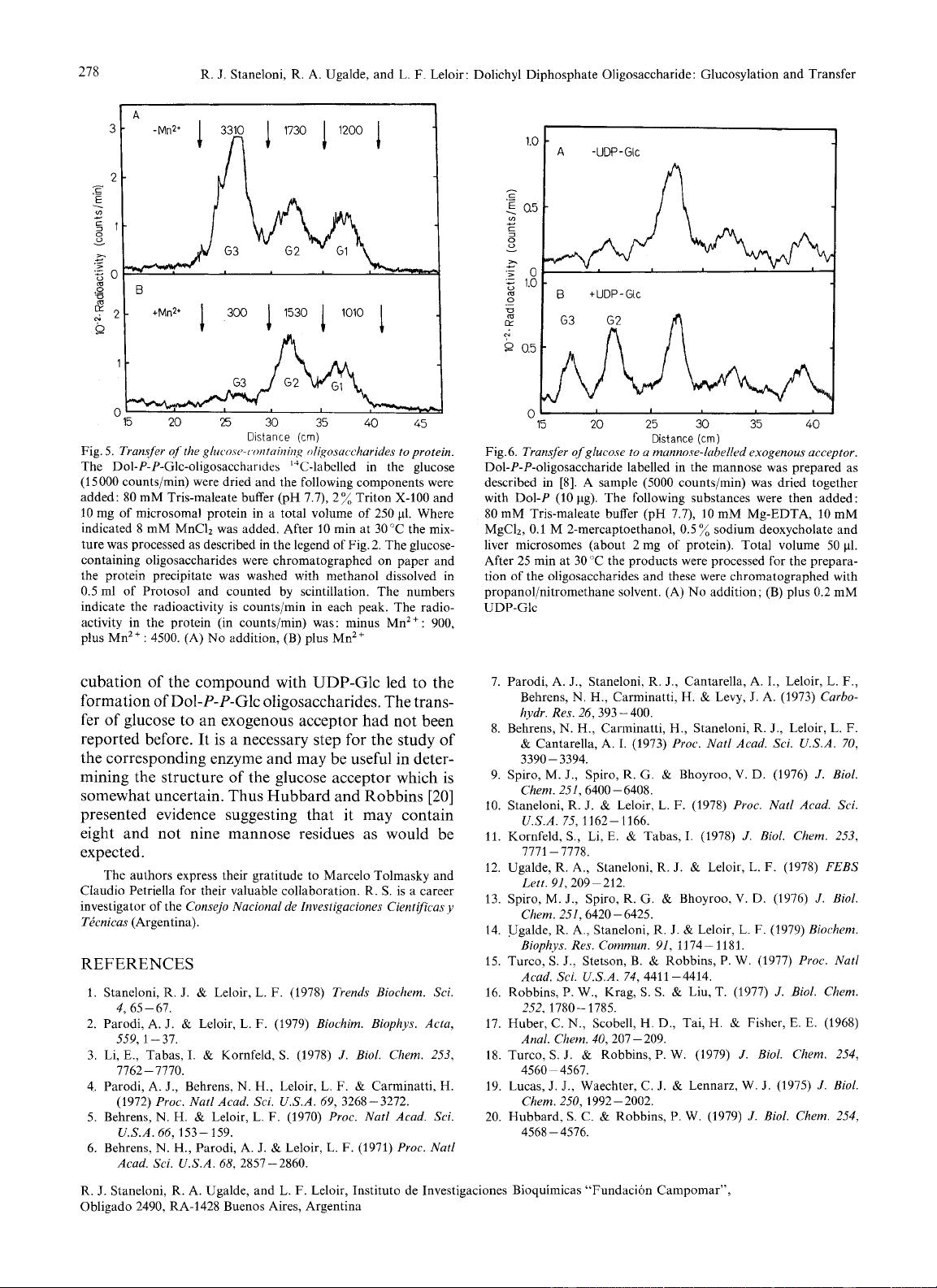
As
shown
in Fig.
5
in the presence of Mn2
+,
when the saccharide
is transferred to protein there was a preferential dis-
appearance of the Glc3-oligosaccharide. This is also
shown in quantitative data (Fig.5). The decrease in
radioactivity in Glc3-oligosaccharide was compensated
by a similar increase in glycoprotein.

R.
J.
Staneloni,
R.
A.
Ugalde, and
L.
F.
Leloir
211
The
Transfer of Glucose to
a
Dolichyl-Diphosphate-
Glucose-Containing Oligosaccharide Precursor. The
formation of
Dol-P-P-Glc-oligosaccharide
has been,
in most studies, carried out by incubating labelled
UDP-Glc with microsomes. These provided an endog-
enous precursor believed
to
contain Man9GlcNAcz
joined to dolichyl diphosphate.
The addition of glucose to a precursor labelled in
the mannose has been tested repeatedly in this labora-
tory but without clear results. Presumably there occurs
a competition between UDP-Glc and GDP-Man for
the Dol-P available.
It was shown by Robbins et al. [16] that incuba-
tion of particulate enzyme preparation of fibroblasts
with GDP-[14C]Man led to the formation of Dol-P-P
oligosaccharides and that these became larger if UDP-
Glc was added in addition. The number of glucose
added was not determined.
Experiments have now been carried out using an
exogenous labelled acceptor. For this purpose liver
microsomes were incubated with GDP-[14C]Man and
the products having the solubility of Dol-P-P-Glc
oligosaccharide were isolated. The major oligosac-
charide formed was found to have a mobility slightly
higher than the Glcl-oligosaccharide. As shown in
Fig. 6 reincubation
of
the compound with microsomes
and UDP-Glc led to the formation of a lipid linked
oligosaccharide whose sugar moiety had the mobility
of Glc3 and Glcz oligosaccharides.
DISCUSSION
The solvent for paper chromatography described
in this paper is nearly the same as the one used by
Huber et al. [17] for thin-layer chromatography.
It
only differs in having slightly more water. With this
solvent maltooligosaccharides of up to 16 units can
be separated in 7days. The neat separation of the
different glucose-containing oligosaccharides has led
to
the clarification of several details of their trans-
formations.
Although Dol-P-P-Glc oligosaccharide was known
to become labelled
on
incubation with radioactive
Dol-P-Glc it remained to be proved
if
the three glu-
coses are introduced by the same mechanism. This
has now been proved. As to the transfer of glucose-
containing oligosaccharide from its Dol-P-P derivative
it had been detected long ago, but at that time the
existence of compounds differing in glucose content
was not known. It has now been found that only the
oligosaccharide containing three glucoses is trans-
ferred to protein. If the dolichyl diphosphate deriva-
tives of Glcz and
Glcl
oligosaccharides are also sub-
strates, the process is
so
slow that it was not detectable
in our experiments. The results complement those of
Turco and Robbins
[18] who isolated the glycopeptide
formed immediately after transfer from Dol-P-P-Glc
-
0
5 10
15
20
25
30
Distance
(cm)
Fig. 3.
Paper chromatography
of
oligosaccharides labelled in the
glucose
and
mannose.
The
preparation was carried out with oviduct
slices
as
described in [9,10]. Chromatography with
the
propanol/
nitromethane solvent for
7
days
1
5
10
15
20
25
30
Distance
(cm)
Fig.
4.
Tramfir
of
glucose
fioni
clolichyl
phosphate
[14CJglucose
to
endogenous acceptors.
The transfer reaction was carried out by
incubation of: 0.1
M
Tris-maleate buffer of pH
7.7,
0.6% Triton
X-100,
10
mM
Na-EDTA,
20 mM 2-mercaptoethano1,
Dol-P-
['4C]Glc
(4000 counts/min dried before the addition of the other
components). Total volume 50
PI,
After 10 min
at
30 "C chloroform
and methanol were added and fractionation
was
carried out as
described in [6]. The oligosaccharides obtained
by
mild acid hydro-
lysis were separated by paper chromatography with the solvent
I-butanol/pyridine/water
(4:3
:4)
for 20 days
oligosaccharide to protein and found that its size cor-
responded to the Gk3-containing compound. Transfer
of mannose-labelled oligosaccharides presumably not
containing glucose has been reported
[8,19].
Their rate
of transfer is slower and may involve endogenous
glucose donors. Further work would be required in
order to find out if the glucose-containing oligo-
saccharides are obligate intermediates in the glycosyla-
tion pathway.
The formation of Dol-P-P-Glc oligosaccharide
has been carried out before by transfer from Dol-P-Glc
to an endogenous acceptor present in the microsomal
preparations [6]. In the experiments reported here an
exogenous acceptor labelled in the mannose was
prepared by incubation of liver microsomes with
GDP[14C]Man followed by solvent extraction.
In-
278
R.
J.
Staneloni,
R.
A. Ugalde, and
L.
F. Leloir: Dolichyl Diphosphate Oligosaccharide: Glucosylation and Transfer
Distance
(crn)
Fig. 5.
Transjer
of
the
gluc~o.cc~-c~r~rr/oirii~i,~
oligosuccharides to protein.
The
Dol-P-P-Glc-oligosaccharides
‘“C-labclled in the glucose
(15000 counts/min) were dried and the following components were
added: 80 mM Tris-maleate buffer (pH 7.7), 2% Triton X-100 and
10
mg of microsomal protein in a total volume of 250
pl.
Where
indicated 8 mM MnCI2 was added. After
10
min at 30
“C
the mix-
ture was processed as described in the legend of Fig. 2. The glucose-
containing oligosaccharides were chromatographed on paper and
the protein precipitate was washed with methanol dissolved in
0.5 ml of Protosol and counted by scintillation. The numbers
indicate the radioactivity is counts/min in each peak. The radio-
activity in the protein (in counts/min) was: minus Mn2+: 900,
plus Mn2+: 4500. (A)
No
addition, (B) plus Mn2+
cubation of the compound with UDP-Glc led
to
the
formation
of
Dol-P-P-Glc oligosaccharides. The trans-
fer
of
glucose to an exogenous acceptor had
not
been
reported before.
It
is
a
necessary step for the study of
the corresponding enzyme and may be useful
in
deter-
mining the structure of the glucose acceptor which
is
somewhat uncertain. Thus Hubbard and Robbins
[20]
presented evidence suggesting that it may contain
eight
and
not nine mannose residues
as
would be
expected.
The authors express their gratitude to Marcel0 Tolmasky and
Claudio Petriella for their valuable collaboration. R.
S.
is a career
investigator
of
the
Consejo Nacional de Investigaciones Cientiji’cas
y
Ticnicas
(Argentina).
REFERENCES
1. Staneloni, R.
J.
&
Leloir, L.
F.
(1978)
Trends Biochem.
Sci.
2. Parodi, A.
J.
&
Leloir, L.
F.
(1979)
Biochim. Biophys. Acta,
3. Li,
E.,
Tabas,
I.
&
Kornfeld,
S.
(1978)
J.
Biol.
Chem.
253,
4. Parodi, A.
J.,
Behrens,
N.
H.,
Leloir, L.
F.
&
Carminatti,
H.
5. Behrens,
N.
H.
&
Leloir, L. F. (1970)
Proc. Narl Acad. Sci.
6.
Behrens,
N.
H.,
Parodi,
A.
J.
&
Leloir,
L.
F.
(1971)
Proc. Natl
4, 65
-
67.
559,l-
37.
7762- 7770.
(1972)
Proc. Natl Acad. Sci.
U.S.A.
69,
3268-3212.
U.S.A.
66,
153-159.
Acad.
Sci. U.S.A.
68,
2857-2860.
’‘O
1
A
-UDP-Glc
0’
I
15
20
25 30 35
40
Distance
(crn)
Fig.
6.
Transfer of glucose to
a
mannore-lubelled exogenous acceptor.
Dol-P-P-oligosaccharide labelled in the mannose was prepared as
described in [8]. A sample
(5000
counts/min) was dried together
with
Dol-P
(10 pg). The following substances were then added:
80 mM Tris-maleate buffer (pH 7.7),
10
mM Mg-EDTA,
10
mM
MgC12,
0.1
M
2-mercaptoethanol, 0.5
%
sodium deoxycholate and
liver microsomes (about
2
mg of protein). Total volume 50
pl.
After 25 min at 30 “C the products were processed for the prepara-
tion
of
the oligosaccharides and these were chromatographed with
propanol/nitromethane solvent. (A)
No
addition; (B)
plus
0.2 mM
UDP-Glc
7. Parodi, A.
J.,
Staneloni, R.
J.,
Cantarella, A.
I.,
Leloir, L.
F.,
Behrens,
N.
H., Carminatti,
H.
&
Levy,
J.
A. (1973)
Carho-
hydr. Res.
26, 393
-
400.
8.
Behrens, N.
H.,
Carminatti,
H.,
Staneloni, R.
J.,
Leloir, L. F.
&
Cantarella,
A.
I.
(1973)
Proc. Natl Acad. Sci. U.S.A.
70,
9. Spiro, M.
J.,
Spiro, R.
G.
91
Bhoyroo,
V.
D. (1976)
J.
Bid.
10. Staneloni, R.
J.
&
Leloir,
L.
F. (1978)
Proc. Natl Acad.
Sci.
11. Kornfeld,
S.,
Li,
E.
&
Tabas,
I.
(1978)
J.
Biol. Chem.
253,
12. Ugalde, R. A,, Staneloni, R.
J.
&
Leloir, L. F. (1978)
FEBS
13. Spiro,
M.
J.,
Spiro,
R.
G.
&
Bhoyroo,
V.
D.
(1976)
J.
Bid.
14. Ugalde, R. A., Staneloni, R.
J.
&
Leloir, L.
F.
(1979)
Biochem.
15. Turco,
S.
J.,
Stetson, B.
&
Robbins,
P.
W. (1977)
Proc.
Natl
16. Robbins, P. W., Krag,
S.
S.
&
Liu, T. (1977)
J.
Biol. Chem.
17. Huber, C. N., Scobell,
H.
D.,
Tai, H.
&
Fisher,
E. E.
(1968)
18. Turco,
S.
J.
&
Robbins, P. W. (1979)
J.
Biol. Chem.
254,
19. Lucas,
J.
J.,
Waechter,
C.
J.
&
Lennarz, W.
J.
(1975)
J.
Bid.
20.
Hubbard,
S.
C.
&
Robbins,
P.
W. (1979)
J.
Biol. Chem.
254,
3390
-
3394.
Chem.
251,6400-6408.
U.S.A.
75, 1162-1166.
7771 -7778.
Lett.
91,209-212.
Chem.
251,6420-6425.
Biophys. Res. Commun.
91, 1174-1181.
Acad. Sci.
U.S.A. 74, 4411 -4414.
252, 1780-1785.
Anal. Chem.
40,207
-
209.
4560-4567.
Chem. 250,
1992
-
2002.
4568 -4576.
R.
J.
Staneloni,
R.
A. Ugalde, and L.
F.
Leloir, Instituto de Investigaciones Bioquimicas “Fundacion Campomar”,
Obligado 2490, RA-1428 Buenos Aires, Argentina