
Circulation Journal Vol.81, July 2017
988 YAZAKI K et al.
Circ J 2017; 81: 988 – 992
doi: 10.1253/circj.CJ-16-1261
Tu et al reported good diagnostic performance of FFR
based on 3-dimensional quantitative CAG (3-D QCA) and
Thrombolysis in Myocardial Infarction (TIMI) frame
counts.
11
The 3-D QCA-based FFR was called quantitative
ow ratio (QFR), which involves an advanced algorithm
that enables fast computation of pressure decreases in
intermediate CAD without any invasive physiologic mea-
surements or pharmacologic hyperemia induction. The
international, multicenter FAVOR pilot study conrmed
the good diagnostic performance of QFR and its designated
software.
15
Contrast-ow QFR, which is based on the
computational hyperemic state and TIMI frame count,
had good correlation and agreement with FFR and similar
diagnostic accuracy to that achieved using the pharmaco-
logic hyperemic state.
The aim of this study was therefore to conrm the appli-
cability of QFR, using wire-based FFR as the reference
standard in the present single-center cohort.
Methods
Study Design
The present study was a retrospective, single-center, obser-
vational study. Patients who underwent CAG and FFR
P
recise assessment of coronary artery disease (CAD),
especially intermediate stenosis, is essential for the
estimation of myocardial ischemia. Anatomic severity
on coronary angiography (CAG) is limited in the assessment
of functional severity.
1,2
Pressure-derived fractional ow
reserve (FFR) for the assessment of myocardial ischemia
has been established because of its availability and feasibility.
Moreover, percutaneous coronary intervention based on
FFR guidance has good prognostic accuracy in intermediate
coronary stenosis.
3–6
Wire-based FFR measurement, how-
ever, has several problems: (1) the hyperemic state is
required, causing chest discomfort; (2) adenosine triphos-
phate, which produces the hyperemic state, cannot be used
in patients with severe asthma, hypotension, or atrio-
ventricular conduction disturbances; and (3) it is challenging
to deliver the pressure wire in tortuous coronary arteries
with complex anatomy. Recently, there have been several
reports on the usefulness of image-based FFR with compu-
tational uid dynamics (CFD).
7–11
Notably, non-invasive
FFR derived from computed tomography CAG (CT-FFR)
along with CFD had better diagnostic performance
compared with the standard anatomical index-based CT
CAG.
9,12–14
As another image-based approach to FFR, com-
puted FFR using CAG data has been introduced.
7,10,11,15
Received December 11, 2016; revised manuscript received February 10, 2017; accepted February 13, 2017; released online March
22, 2017 Time for primary review: 25 days
Department of Cardiology, Cardiovascular Center, Ogikubo Hospital, Tokyo, Japan
Mailing address: Kyoichiro Yazaki, MD, Department of Cardiology, Cardiovascular Center, Ogikubo Hospital, 3-1-24 Imagawa,
Suginami-ku, Tokyo 167-0035, Japan. E-mail: kamisamakaranookurimono@gmail.com
ISSN-1346-9843 All rights are reserved to the Japanese Circulation Society. For permissions, please e-mail: cj@j-circ.or.jp
Applicability of 3-Dimensional Quantitative Coronary
Angiography-Derived Computed Fractional Flow Reserve for
Intermediate Coronary Stenosis
Kyoichiro Yazaki, MD; Masato Otsuka, MD, PhD; Shohei Kataoka, MD;
Mitsuru Kahata, MD; Asako Kumagai, MD; Koji Inoue, MD, PhD;
Hiroshi Koganei, MD, PhD; Kenji Enta, MD, PhD; Yasuhiro Ishii, MD, PhD
Background: Quantitative flow ratio (QFR) is a newly developed image-based index for estimating fractional flow reserve (FFR).
Methods and Results: We analyzed 151 coronary arteries with intermediate stenosis in 142 patients undergoing wire-based FFR
measurement using dedicated software. Predefined contrast flow QFR, which was derived from 3-dimensional quantitative coronary
angiography (3-D QCA) withThrombolysis in Myocardial Infarction (TIMI) frame counts, was compared with FFR as a reference. QFR
had good correlation (r=0.80, P<0.0001) and agreement (mean difference: 0.01±0.05) with FFR. After applying the FFR cut-off ≤0.8,
the overall accuracy rate of QFR ≤0.8 was 88.0%. On receiver operating characteristics analysis, the area under the curve was 0.93
for QFR. In contrast, 3-D QCA-derived anatomical indices had insufficient correlation with FFR and diagnostic performance compared
with QFR.
Conclusions: QFR had good correlation and agreement with FFR and high diagnostic performance in the evaluation of intermediate
coronary stenosis, suggesting that QFR may be an alternative tool for estimating myocardial ischemia.
Key Words: 3-dimensional quantitative coronary angiography; Fractional flow reserve; Myocardial ischemia; Quantitative flow ratio
ORIGINAL ARTICLE
Cardiovascular Intervention

Circulation Journal Vol.81, July 2017
9893-D QCA-Derived Computed FFR
Wire-Based FFR
FFR was measured in all patients using a coronary pressure
wire (Aeris
TM
; St. Jude Medical, St. Paul, MN, USA; and
Verrata
®
; Volcano, San Diego, CA, USA). After calibration
and equalization, the pressure wire was advanced distally
to the stenosis. Maximum hyperemia was induced with i.v.
adenosine triphosphate at a concentration of 150–180 µg/
kg/min. Both the distal coronary pressure at the pressure
sensor and the proximal pressure at the end-hole of the
catheter were recorded simultaneously. The pressure sensor
was pulled back to the catheter tip to check or correct the
pressure drift (Figure 1C).
Calculation of QFR
Following 3-D QCA reconstruction, QFR was calculated
using the same software. QFR was based on the following
principles: (1) coronary pressure does not decrease unless
stenosis exists;
16
(2) coronary ow determines the pressure
decrease using the uid dynamics equation;
17
(3) stenosis
characteristics are determined by the deviation of the ste-
nosis lesion with respect to the reference size; and (4) mass
ow rate depends on the mean ow velocity and reference
size at each point. In the present study, the contrast-ow
QFR pullback was adopted: TIMI frame count was mea-
sured on CAG and the coronary ow velocity (CFV) was
used as the modeled hyperemic ow velocity (HFV).
The relationship between HFV and CFV has been
reported previously using several equations. After calculating
HFV, integration of the pressure decrease of all subseg-
ments proximal to that interrogated location results in the
QFR at the arbitrary position. The detailed algorithm and
theory of QFR computation have been described in a
previous report.
15
Examples of reconstructed 3-D QCA and
measured QFR are shown in Figure 1D. Lumen diameter
pullback and QFR are shown in Figure 1E.
Measurement and Analysis
Wire-based FFR and QFR were analyzed and validated in
measurements were enrolled. 3-D QCA with QFR recon-
structed from CAG was analyzed and compared with FFR
as a reference in a core laboratory at the same institute.
This study was conducted after receiving ethics approval
of the institutional review board. All patients provided
written informed consent before enrollment.
Subjects and Target Lesions
One hundred and seventy-one consecutive vessels in 156
patients who underwent CAG and subsequent FFR mea-
surement between May 2014 and July 2016 were screened.
Exclusion criteria were as follows: (1) lack of 2 optimal
angiographic projections at least 25° apart; (2) overlapping
interrogated vessels with too much shortening without
preferred references in proximal or distal vessels; (3) insuf-
cient injected contrast for QCA analysis; and (4) location
of the target lesion at the ostium of the left or right coronary
artery.
3-D QCA Reconstructed With Angiography
CAG was performed using the monoplane or biplane
X-ray system (Innix Celeve-i INFX-8000V/8000C; Toshiba
Medical Systems, Tokyo, Japan). These images were
recorded at 15frames/s. The 3-D images were reconstructed
using validated software (QAngio
®
XA/3-D; Medis, Leiden,
the Netherlands) by selected investigators who were blinded
to FFR. Initially, we selected 2 angiographic projections
>25° apart (Figure 1A,B). Subsequently, we registered
proximal and distal points as the references and the regions
of interest. The 3-D QCA was reconstructed with the
proposed analytic model, which was based on geometrical
features derived from the entrance angle of coronary
stenosis, angularity of the center line, and reference points
of the lumen diameter. The percent diameter stenosis
(DS%), lesion length, minimum lumen diameter (MLD),
reference proximal and distal vessel diameters, and percent
area stenosis (AS%) were calculated using 3-D QCA.
Figure 1. Quantitative flow ratio (QFR)
analysis of intermediate stenosis of the
left anterior descending artery (LAD).
(A) Right anterior oblique (RAO) coro-
nary angiograms of the left coronary
artery at 45° with a cranial (CRA) angle
of 45° and (B) RAO projection of 30°.
(C) Fractional flow reserve (FFR) was
0.72. (D) QFR was calculated as 0.72
(yellow asterisk; A and B, red asterisks).
(E) Lumen diameter and QFR pullback.

Circulation Journal Vol.81, July 2017
990 YAZAKI K et al.
The Youden index was used to identify the best cut-o for
every index; the 3-D QCA-derived index and QFR were
used for predicting functionally signicant stenosis. All
statistical analysis was performed using JMP
®
12 (SAS
Institute, Cary, NC, USA).
Results
Baseline Characteristics
A total of 20 vessels, which accounted for 12% of all vessels,
were excluded due to the aforementioned exclusion criteria
for the selection of the 2 optimal CAG images. The remaining
151 vessels in 142 patients underwent QFR analysis. Patient
and lesion characteristics are listed in Tables 1,2. Average
DS%, lesion length, MLD, reference diameter, and AS%
the core laboratory of the present institute. QFR calculation
was performed by the selected investigators who were
blinded to the results of wire-based FFR.
Statistical Analysis
Normally distributed continuous variables are expressed
as mean ± SD and non-normal variables as median (IQR).
Categorical variables are expressed as percentages. Data
were analyzed on a per-patient basis for clinical character-
istics and on a per-vessel basis for the remaining calcula-
tions. Pearson correlation was used to quantify the
correlation between FFR and QFR; Spearman correlation
was used to quantify the correlations between FFR and
indices of 3-D QCA. Agreement between FFR and QFR
was assessed on Bland-Altman plot. The performance of
QFR for predicting functionally signicant stenosis was
assessed using sensitivity, specicity, positive predictive
value (PPV), negative predictive value (NPV), and diag-
nostic accuracy. The area under the curve (AUC) on
receiver operating characteristic (ROC) analysis was used
to assess the diagnostic accuracy of QFR and 3-D QCA.
Table 1. Baseline Patient Characteristics
Characteristics
Mean age (years) 72.5±9.5
Male 100 (70.4)
Mean BMI (kg/m
2
) 23.9±3.2
Current smoking 33 (23.2)
Hypertension 101 (71.1)
Hyperlipidemia 88 (62.0)
Diabetes 41 (28.9)
Cardiovascular history
Prior PCI 58 (40.8)
Prior CABG 2 (1.4)
Prior MI 30 (21.2)
CAD type
Stable angina 72 (50.7)
UA/NSTEMI 1 (0.7)
Asymptomatic CAD 69 (48.6)
Data given as mean ± SD or n (%). BMI, body mass index; CABG,
coronary artery bypass grafting; CAD, coronary artery disease;
MI, myocardial infarction; NSTEMI, non-ST-elevated myocardial
infarction; PCI, percutaneous coronary intervention; UA, unstable
angina.
Table 2. Baseline Lesion Characteristics
Lesion characteristics
Index artery
Left anterior descending artery 96 (63.6)
Left circumflex artery 25 (16.6)
Right coronary artery 26 (17.2)
Diagonal artery 2 (1.3)
Saphenous vein graft 1 (0.7)
Left main trunk 1 (0.7)
Bifurcation lesions 71 (47.0)
Stented lesion 21 (13.9)
Tandem/diffuse lesion 47 (31.1)
Fractional flow reserve
Mean ± SD 0.84±0.08
Median (IQR) 0.85 (0.79–0.92)
Percent diameter stenosis 48.8±8.2
Percent area stenosis 62.2±11.9
Minimum lumen diameter (mm) 1.38±0.39
Reference vessel diameter (mm) 2.84±0.57
Lesion length (mm) 16.8 (12.1–24.6)
Severity of CS (ACC/AHA classification)
50% 64 (42.4)
75% 87 (57.6)
Data given as n (%), mean ± SD, or median (IQR). Anatomical
parameters were quantified on 3-dimensional quantitative coronary
angiography. ACC/AHA, American College of Cardiology/American
Heart Association; CS, coronary stenosis.
Figure 2. Correlation and agreement of quantitative flow ratio (QFR) with the conventional wire-based fractional flow reserve (FFR).
Circulation Journal Vol.81, July 2017
9913-D QCA-Derived Computed FFR
performance.
7,13
Tu et al took <10 min to calculate 3-D
QCA-based FFR using the newly developed algorithm.
11
We also achieved a shorter QFR analysis time of, at most,
590 s (median, 266 s; IQR, 181–332 s), which was an accept-
able time to calculate values. Moreover, contrast-ow
QFR (which is based on computational hyperemic state
and TIMI frame count) used in the present study had good
correlation and agreement with wire-based FFR and accept-
able diagnostic performance. Tu et al reported that QFR
based on the pharmacologically induced hyperemic state did
not have better diagnostic performance than contrast-ow
QFR,
15
which might be explained by the following: (1) the
contrast could induce submaximum hyperemia similar to
the pharmacologically induced state; (2) CFV could be
accelerated by pharmacological hyperemia, which could
lead to underestimation of functional ischemia; and (3)
deterioration of angiographic images induced by pharma-
cological hyperemia with increasing heart beat and ow
velocity. We did not analyze QFR based on pharmacologic
hyperemia in the present study; therefore, we could not
compare contrast-ow QFR and QFR based on pharmaco-
logic hyperemia. We were, however, able to determine an
acceptable value of QFR with regard to diagnostic accuracy,
correlation, and agreement with FFR, as Tu et al demon-
strated, thereby demonstrating the feasibility of QFR.
of interrogated vessels were 48.8±8.2%, 19.79±10.65 mm,
1.45±0.95 mm, 2.84±0.57 mm, and 62.2±11.9%, respectively.
The measured FFR was 0.84±0.08 and abnormal FFR
≤0.8 was recorded for 46 vessels (30.5%).
Correlation With FFR
Average QFR was 0.84±0.07. QFR had good correlation
(r=0.80, P<0.0001) and agreement (mean dierence,
0.01±0.05) with FFR (Figure 2). In contrast, other ana-
tomical indices such as AS%, DS%, and MLD had weaker
correlations (ρ=−0.26, P=0.001; ρ=−0.37, P<0.0001; and
ρ=0.52, P<0.0001) with FFR than QFR.
Accuracy of QFR
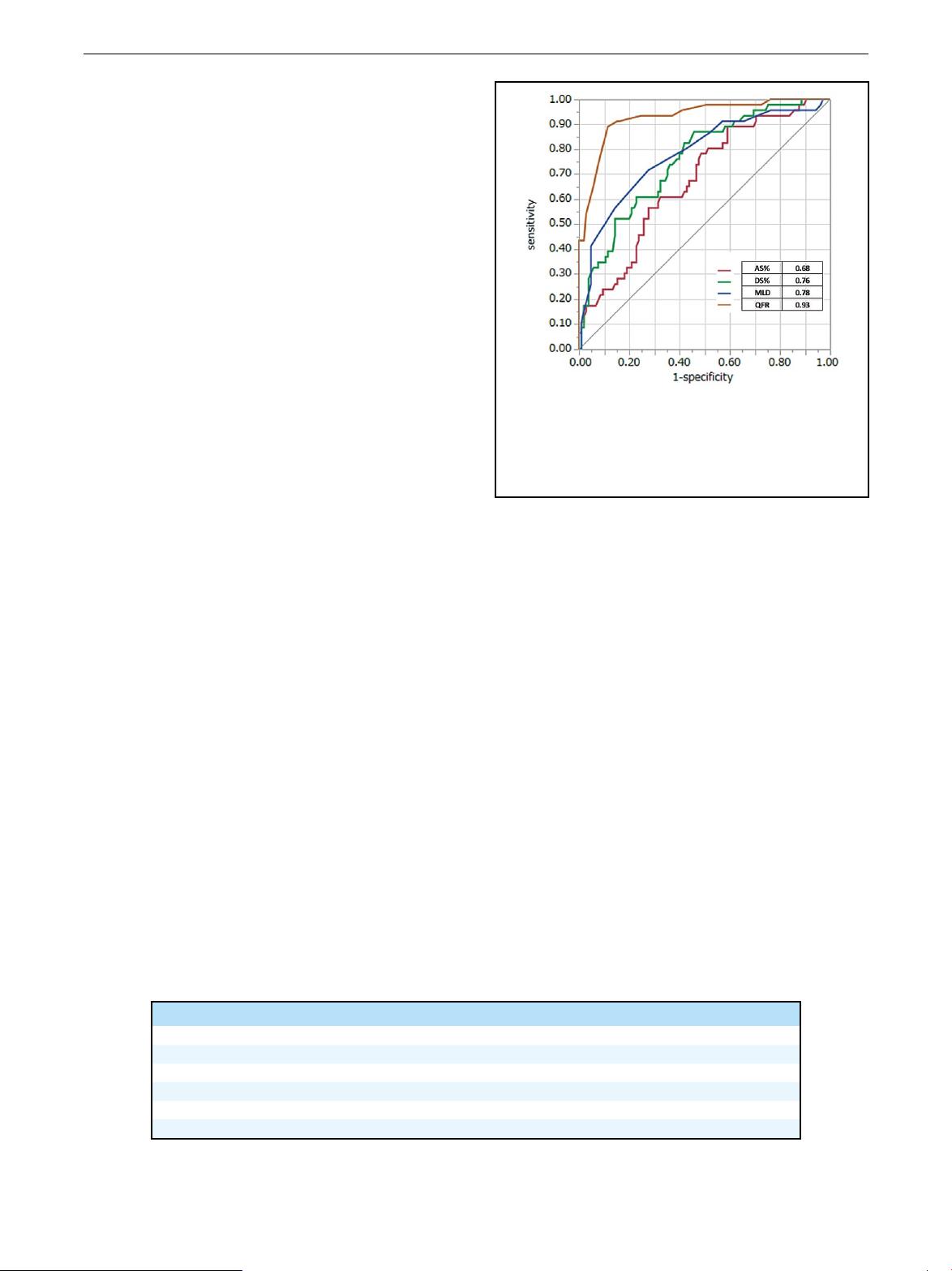
On ROC analysis with an FFR cut-o ≤0.8, QFR had a
greater AUC (0.93) than DS% (AUC, 0.76), MLD (AUC,
0.78), and AS% (AUC, 0.68; Figure 3). Applying the FFR
cut-o ≤0.8 to QFR ≤0.8 resulted in 41 true-positive, 93
true-negative, 12 false-positive, and 5 false-negative results.
The accuracy, sensitivity, specicity, PPV, and NPV of QFR
were 88.7%, 89.1%, 88.6%, 77.4%, and 94.9%, respectively
(Table 3).
Calculation of QFR
The average time to calculate QFR was 266 s (IQR, 181–
332 s). This included time spent selecting 2 optimal angio-
graphic images to complete the QFR calculation.
Discussion
Main Findings
QFR had good correlation (r=0.80, P<0.0001) and agree-
ment (mean dierence: 0.01±0.05) with wire-based FFR.
When FFR ≤0.8 was used as the cut-o for diagnosis of
myocardial ischemia, QFR had larger AUC of 0.93 on
ROC analysis than did 3-D QCA-derived anatomical indices.
When the QFR cut-o was also set at 0.8, it provided
acceptable diagnostic performance (sensitivity, specicity,
PPV, and NPV: 88.7%, 89.1%, 88.6%, 77.4%, and 94.9%).
Moreover, QFR calculation required a relatively short
time for the entire analysis.
Previous Modalities
CFD simulation has contributed to virtual FFR computa-
tion (e.g., CT-FFR, 3-D QCA-based FFR), as previously
reported.
9,11
CFD plays an important role in reconstructing
coronary trees and enables the recognition of functional
severity in intermediate stenosis. Complex computation,
however, requires a longer time to create a precise map of
the coronary tree. Complete analysis of CT-FFR and cal-
culation of rotational angiography-based FFR, based on
CFD, take at least several hours despite the good diagnostic
Figure 3. Receiver operating characteristics curve with
fractional flow reserve cut-off ≤0.8. Area under the curve was
significantly greater for the quantitative flow ratio (QFR) com-
putation model compared with the 3-dimensional anatomical
indices. AS%, percent area stenosis; DS%, percent diameter
stenosis; MLD, minimum lumen diameter.
Table 3. Diagnostic Performance of QFR and 3-Dimensional QCA Anatomical Indices for FFR ≤0.8
Diagnostic measure QFR ≤0.8 DS% ≥47.5% AS% ≥58% MLD ≤1.20
Diagnostic accuracy (%) 88.7 64.2 55.6 72.2
Sensitivity (%) 89.1 87.0 89.1 71.7
Specificity (%) 88.6 54.3 41.0 72.4
PPV (%) 77.4 40.5 39.8 53.2
NPV (%) 94.9 90.5 89.6 85.4
AS%, percent area stenosis; DS%, percent diameter stenosis; FFR, fractional flow reserve; MLD, minimum lumen
diameter; NPV, negative predictive value; PPV, positive predictive value; QCA, quantitative coronary angiography;
QFR, quantitative flow ratio.

Circulation Journal Vol.81, July 2017
992 YAZAKI K et al.
2010; 55: 173 – 185.
2. Tonino PAL, De Bruyne B, Pijls NHJ, Siebert U, Ikeno F, van’t
Veer M, et al. Fractional ow reserve versus angiography for
guiding percutaneous coronary intervention. N Engl J Med 2009;
360: 213 – 224.
3. van Nunen LX, Zimmermann FM, Tonino PAL, Barbato E,
Baumbach A, Engstrøm T, et al. Fractional ow reserve versus
angiography for guidance of PCI in patients with multivessel
coronary artery disease (FAME): 5-year follow-up of a ran-
domised controlled trial. Lancet 2015; 386: 1853 – 1860.
4. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG,
Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS
focused update of the guideline for the diagnosis and management
of patients with stable ischemic heart disease: A report of the
American College of Cardiology/American Heart Association
Task Force on Practice Guidelines, and the American Association
for Thoracic Surgery, Preventive Cardiovascular Nurses Associa-
tion, Society for Cardiovascular Angiography and Interventions,
and Society of Thoracic Surgeons. J Am Coll Cardiol 2014; 64:
1929 – 1949.
5. Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E,
Bech JW, van’t Veer M, et al. Percutaneous coronary intervention
of functionally nonsignicant stenosis: 5-year follow-up of the
DEFER Study. J Am Coll Cardiol 2007; 49: 2105 – 2111.
6. Pijls NHJ. Fractional ow reserve to guide coronary revascular-
ization. Circ J 2013; 77: 561 – 569.
7. Morris PD, Ryan D, Morton AC, Lycett R, Lawford PV, Hose
DR, et al. Virtual fractional ow reserve from coronary angiog-
raphy: Modeling the signicance of coronary lesions: Results
from the VIRTU-1 (VIRTUal Fractional Flow Reserve From
Coronary Angiography) study. JACC Cardiovasc Interv 2013; 6:
149 – 157.
8. Morris PD, van de Vosse FN, Lawford PV, Hose DR, Gunn JP.
“Virtual” (computed) fractional ow reserve: Current challenges
and limitations. JACC Cardiovasc Interv 2015; 8: 1009 – 1017.
9. Norgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et
al. Diagnostic performance of noninvasive fractional ow reserve
derived from coronary computed tomography angiography in
suspected coronary artery disease: The NXT trial (Analysis of
Coronary Blood Flow Using CT Angiography: Next Steps). J
Am Coll Cardiol 2014; 63: 1145 – 1155.
10. Papafaklis MI, Muramatsu T, Ishibashi Y, Lakkas LS, Nakatani
S, Bourantas CV, et al. Fast virtual functional assessment of
intermediate coronary lesions using routine angiographic data
and blood ow simulation in humans: Comparison with pressure
wire: Fractional ow reserve. EuroIntervention 2014; 10: 574 – 583.
11. Tu S, Barbato E, Koszegi Z, Yang J, Sun Z, Holm NR, et al.
Fractional ow reserve calculation from 3-dimensional quantita-
tive coronary angiography and TIMI frame count: A fast
computer model to quantify the functional signicance of
moderately obstructed coronary arteries. JACC Cardiovasc Interv
2014; 7: 768 – 777.
12. Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, et
al. Diagnosis of ischemia-causing coronary stenoses by noninva-
sive fractional ow reserve computed from coronary computed
tomographic angiograms. Results from the prospective multi-
center DISCOVER-FLOW (Diagnosis of Ischemia-Causing
Stenoses Obtained Via Noninvasive Fractional Flow Reserve)
study. J Am Coll Cardiol 2011; 58: 1989 – 1997.
13. Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van
Mieghem C, et al. Diagnostic accuracy of fractional ow reserve
from anatomic CT angiography. JAMA 2012; 308: 1237 – 1245.
14. Nakazato R, Park HB, Berman DS, Gransar H, Koo BK, Erglis
A, et al. Noninvasive fractional ow reserve derived from
computed tomography angiography for coronary lesions of
intermediate stenosis severity: Results from the DeFACTO study.
Circ Cardiovasc Imaging 2013; 6: 881 – 889.
15. Tu S, Westra J, Yang J, von Birgelen C, Ferrara A, Pellicano M,
et al. Diagnostic accuracy of fast computational approaches to
derive fractional ow reserve from diagnostic coronary angiog-
raphy: The International Multicenter FAVOR Pilot Study.
JACC Cardiovasc Interv 2016; 9: 2024 – 2035.
16. De Bruyne B, Paulus WJ, Pijls NHJ. Rationale and application
of coronary transstenotic pressure gradient measurements. Cathet
Cardiovasc Diagn 1994; 33: 250 – 261.
17. Kirkeeide RL. Coronary obstructions, morphology and physi-
ologic signicance. In: Reiber JHC, Serruys PW, editors. Quanti-
tative coronary arteriography. Dordrecht: Springer Netherlands,
1991; 229 – 244.
Clinical Implications of QFR
QFR analysis required less evaluation time and, hence,
could be used for online analysis. Furthermore, online QFR
may have the potential to augment the information on
individual functional severity in multiple intermediate
coronary stenosis, which may be utilized in coronary revas-
cularization. To utilize QFR for a more accurate clinical
evaluation, we should take into account the wide variation
in agreement between QFR and FFR at the approximate
value of 0.8. It is possible that the hybrid strategy might
partially solve the problem based on subanalysis of the
present cohort. When limited lesions with QFR <0.75 or
>0.85 were examined (n=85), the diagnostic performance
of QFR for FFR ≤0.8 was excellent, with diagnostic accu-
racy, sensitivity, specicity, PPV, and NPV of 96%, 84%,
100%, 100%, and 96%, respectively. Using this proposed
hybrid method, FFR should be measured in lesions with
QFR 0.75–0.85. This may have the potential to be a
sophisticated method of evaluation for myocardial ischemia
using both QFR and FFR.
Study Limitations
This study was a retrospective, observational study involving
a small sample size at a single center. Because of the retro-
spective analysis of CAG, we could not always obtain the
appropriate images for reconstruction of 3-D QCA, which
partially inuenced patient selection. As a result, 12% of all
vessels were excluded due to the absence of optimal angi-
ography. Moreover, we could not validate the interobserver
or intraobserver variability in these selected vessels. It is
dicult to recommend the practical use of QFR for esti-
mating myocardial ischemia due to the following reasons:
(1) QFR could require more contrast agent to achieve
optimal angiographic visualization; (2) it is unclear whether
QFR can yield precise values independent of lesion char-
acteristics or vessel characteristics; and (3) in the present
study, only intermediate stenosis was included; therefore,
we could not conrm that the outcome is independent of
lesion severity. We enrolled various consecutive targets,
including bifurcation and tandem/diuse or stented lesions
in the main epicardial artery, bypass graft, or side branches
to exclude selection bias. This enrolling of consecutive
targets suggests the potential benet of QFR for lesions
evaluated using only angiography, which may also be
attractive for catheter interventionists.
Conclusions
QFR had good correlation and agreement with wire-based
FFR and high diagnostic performance in the evaluation of
intermediate coronary stenosis, suggesting its potential as
an alternative tool for estimating myocardial ischemia.
Acknowledgments
We thank Editage (www.editage.jp) for English language editing.
Funding
None.
Disclosures
The authors declare no conict of interest.
References
1. Kern MJ, Samady H. Current concepts of integrated coronary
physiology in the catheterization laboratory. J Am Coll Cardiol