














205 citations
...Perhaps these strains could serve as useful sources of TPA catabolic genes for synthetic biology efforts associated with biological plastics recycling and upcycling (67)....
[...]
186 citations
155 citations
69 citations
45 citations
...Samples from both time zero and the endpoints are grown under selective conditions, after which their gDNA is extracted (2), barcodes are amplified from conserved priming sites (3), barcode abundance is calculated via Illumina sequencing (4), and gene fitness per condition is calculated by comparing the relative abundances of mutants before and after selection (5)....
[...]
33 citations
32 citations
23 citations
11 citations
10 citations
Currently further metagenome and mechanistic studies of this important enzyme class are carried out by the scientific community, most likely discovering protein family members with superb activities or at least interesting amino acid variations. Accessing non-biodegradable plastics of petrochemical origin ( and in the future of biological origin ) as carbon source for fermentations enables biotechnology to valorize enormous waste streams for the sustainable production of many valuable products by exploiting the metabolic versatility of microorganisms. While the mesophilic PET hydrolase from I. sakaiensis15 suggests consolidated hydrolysis and utilization, the authors focused on sequential plastic depolymerization and monomer conversion on purpose.
The use of enzyme cocktails will also enable feedstock flexibility, especially when combined with microbes engineered to accept other plastic-derived substrates.
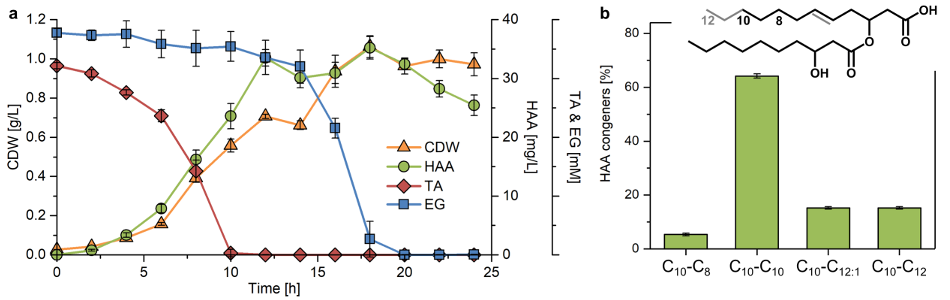
Since an isocyanate moiety can react with both an hydroxyl and a carboxylic acid group, and HAA is an hydroxy acid, its direct polymerization with 4,4’- methylene diphenyl diisocyanate (MDI) and butanediol (BDO) was performed and led to the formation of a poly(amide urethane).
The polymer started to degrade and to lose volatile products at 160 °C and then showed a multi-step degradation profile with the main mass loss occurring between 250 and 350 °C.
Renewable plastics including PHA have already been proposed to effectuate a shift of the packaging industry, which consumes over 38% of the plastics produced53.
Lignocellulose-derived substrates come with a large fraction of solids, which are not completely degraded impeding the application of, e.g., enzyme immobilization or in situ removal of formed monomers (by e.g., precipitation or extraction).
While plastic, due to its lightweight and sturdiness, has many environmentally beneficial applications, the environmental damage caused by plastic must be arrested by addressing the end-of-life challenge.
Three 2 ml samples were taken at regular intervals for the analysis of TA, EG and nitrogen concentrations, biomass, and PHA accumulation for each time point.
An alternative way to increase plastic recycling is to add additional value to the plastic waste, not aiming for the same material or consumer good (e.g., bottle-to-bottle recycling), but rather upcycling to chemicals and materials of higher value.
The use of enzyme engineering and enzyme cocktail formulation will enable an even more efficient PET degradation, for instance using specialized enzymes of the various types of PET, i.e. high molecular weight PET and PET oligomers mono-(2-hydroxyethyl)TA (MHET) and bis2(hydroxyethyl)TA (BHET); possibly combined with chemical hydrolysis methods such as glycolysis56.
The concentration of TA and EG showed a steep near-linear increase within the first 24 h of the hydrolytic reaction and weakened to a markedly lower rate until 120 h.
TT supervised the experiments regarding monomer metabolism and HAA synthesis, drafted the manuscript, and coordinated the study, TN provided strain Pseudomonas sp. GO16, supervised the experiments regarding PHA synthesis and drafted parts of the manuscript, RW supervised the experiments regarding depolymerization and drafted parts of the manuscript, EP supervised the experiments regarding polymerization and drafted parts of the manuscript, KS carried out the experiments regarding monomer metabolism and HAA synthesis, NB carried out the experiments regarding PHA synthesis, AH carried out the experiments regarding depolymerization, MJ carried out the experiments regarding polymerization, SK was involved in PHA bioprocess design, NW was involved in designing and coordinating the study, drafted parts of the manuscript and critically read the manuscript, RP was involved in designing the study and critically read the manuscript, LA was involved in designing the study and critically read the manuscript, WZ was involved in designing the study and critically read the manuscript, KOC designed the study and critically read the manuscript, LMB designed and coordinated the study and critically read the manuscript.
For obvious reasons, the biodegradation of these recalcitrant plastics are exciting discoveries that give hope for the natural bioremediation of sites contaminated with plastic waste in the environment, although plastic degradation in the ocean seems to be slow at best and the anthropogenic dissemination of new plastic pollution likely far exceeds its decay18.
In 2018, 359 million tons of plastics have been produced worldwide and this number is growing at a rate of approximately 3% per annum1.