




Did you find this useful? Give us your feedback












47 citations
39 citations
34 citations
10 citations
7 citations
1,980 citations
1,438 citations
942 citations
721 citations
710 citations
These antibodies could still have important effector potential through mechanisms such as antibody-directed cell cytotoxicity and further studies to examine the specificity and function of the SARS-CoV-2 B cell repertoire in children following natural infection or vaccination would be of value.
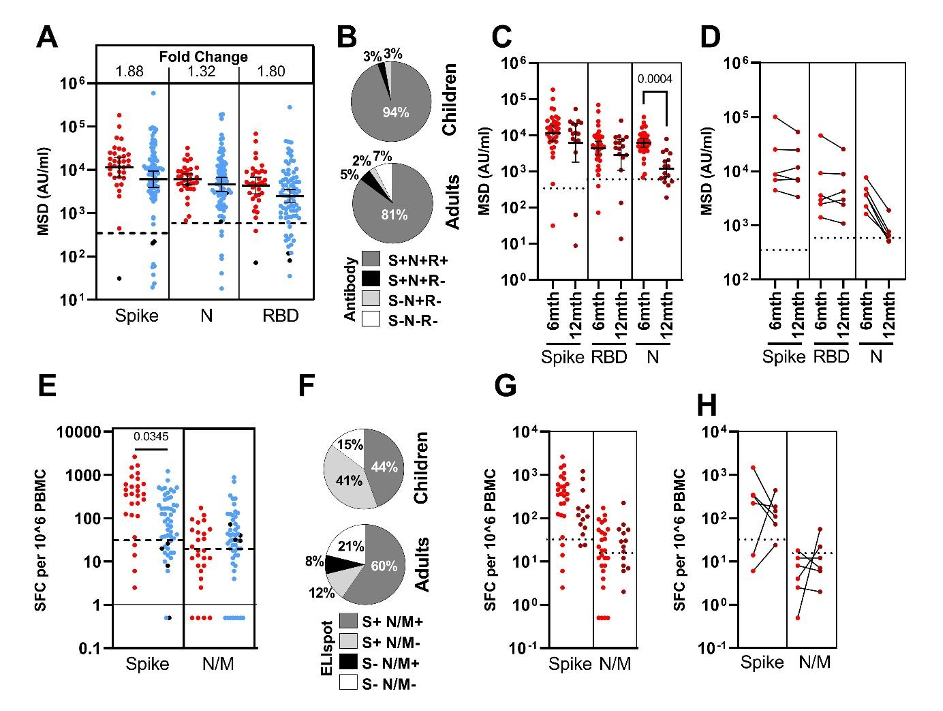
Spike-specific antibody responses were also largely maintained in children at 12 months, whereas responses against nucleocapsid showed significant waning.
Children develop robust and broad antibody responses after SARS-CoV-2 infection Blood samples were obtained from 91 children and 154 adults, including 35 children and 81 adults known to be seropositive in previous rounds of testing.
To examine the presence of cross-reactive T cells in seronegative children the authors hypothesised that expansion of T cells in response to SARS-CoV-2 would be associated with an associated increase in HCoV-reactive responses.
The SARS-CoV-2 pandemic has resulted in over 4.2 million deaths to date and the most significant determinant of outcome is age at the time of primary infection1.
Blood tubes were spun at 300g for 10mins prior to removal of plasma which was then spun again at 800g for 10mins and stored at -80°C.
HEK293T cells were transfected with the appropriate SARS-CoV-2 S gene expression vector in conjunction with lentiviral vectors p8.91 and pCSFLW using polyethylenimine (PEI, Polysciences, Warrington, USA).
T cell responses were detectable in more than half the seronegative children, including samples taken pre-pandemic, and are likely to represent HCoV-specific T cell responses that cross react against SARS-CoV2 peptides 34, 35.
One unique feature of SARS-CoV-2 infection in children is the development of a rare complication known as pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS), also known as paediatric multisystem inflammatory syndrome (MIS-C), which shares features with Kawasaki disease and toxic shock syndrome 7, 8.
The S2 domain, however, selectively reduced antibody binding to the two HCoV Beta-coronaviruses, OC43 and HKU-1 (p <0.0001 and p=0.0014, respectively by repeated measure one-way ANOVA with Holm-Sidak’s multiple comparison test).
The authors find that the virus-specific T cell response is higher in children compared to adults and this mirrored the humoral response in that responses against the spike protein were markedly increased compared to nucleocapsid and envelope proteins.
These comprise the Beta-coronaviruses OC43 and HKU-1, which have 38% and 35% amino acid homology with SARS-CoV-2, as well as the more distantly related Alphacoronaviruses NL63 and 229E, each with around 31% homology 11.
The S2 domain is more highly conserved between HCoV than S1 and this pattern is compatible with preferential targeting of structurally-conserved epitopes by HCoV-specific antibodies in children 30 with the potential for neutralising activity against SARS-CoV-2 15, 31.
In conclusion, the authors show that children display a characteristic robust and sustained adaptive immune response against SARS-CoV-2 with substantial cross-reactivity against other HCoV.
Of note, young adults have been recently reported to have higher T cellresponses to HCoV than older people and these can cross-react with SARS-CoV-2 36.
to definitively assess the presence of cross-reactive T cells in children the authors obtained pre-pandemic PBMC from children and observed that 50% of these had notable responses to spike by ELISpot but lacked cellular responses against N/M peptides.
plasma or positive control antibody were pre-incubated with biotinylated-Fc-chimera-S1-RBD protein prior to addition of bead bound ACE-2.