




Did you find this useful? Give us your feedback











38 citations
24 citations
5 citations
9 citations
8 citations
Thermogravimetry is one of the most used techniques to study the kinetics of absorption and desorption of hydrogen from Mg related compounds.20-23 Authors normally employ conventional constant heating rate or isothermal experiments to collect the data.
Solid-state hydrides, including metal, intermetallic and complex hydrides present the highest volumetric capacities of hydrogen storage, and have recently attracted interest for thermal energy storage applications.
Magnesium needs temperatures above 573 K to absorb hydrogen, the dehydrogenation temperature of MgH2 is even higher because of its high thermodynamic stability, and finally, MgH2 presents a high reactivity towards air and oxygen.3, 7, 12-13 Desorption temperature has been reduced and the hydrogenationdehydrogenation reactions have been fasten by mechanical milling and alloying, doping with catalytic additives and employing cycles of hydrogenation-dehydrogenation.11, 14-19 However, the mechanism and kinetic parameters of these reactions, which are of the most interest for practical applications, have been less thoroughly studied.
The objective of this work is the application of the CRTA methodology for the first time to study the dehydrogenation kinetics of MgH2 in conditions far from equilibrium.
The use of the CRTA procedure is not limited to first order reactions, but it can be employed independently of the kinetic model followed by the reaction, and for this reason has been used to study the kinetics of thermal decomposition ofdifferent materials.
The kinetic parameters obtained from the combined kinetic analysis were tested simulating the CRTA curves and the curve registered at 2.5 K min-1 heating rate, assuming such kinetic parameters.
61-62 The results here reported have been obtained under high vacuum in order to assure that the dehydrogenation of magnesium hydride is taking place very far from equilibrium and thus the activation energy obtained is representative of the forward reaction.
Among all the solid-state hydrides, Mg-based is the most studied family, due to the large hydrogen content of MgH2 (7.6 mass%), the high hydrogenationdehydrogenation enthalpy and the ample abundance of magnesium in earth.7-11 Nevertheless, the kinetic and thermodynamic properties of Mg-based materials present several issues that have to be overcome for its use in practical applications.
If the α-T (or α-t) plot is obtained at a constant decomposition rate (C = dα/dt), equation (2) can be rearranged, after taking logarithms, in the form:ln ln 3It has been previously shown that CRTA permits to discriminate the kinetic model obeyed by the reaction from the analysis of a single α-T plot, which is not possible if this plot is obtained from conventional rising temperature experiments.
The combined kinetic analysis, which allows calculating the kinetic parameters without any assumption about the kinetic model followed by the reaction, has been applied to the curves registered under CRTA conditions together with a curve registered under linear heating rate conditions, and the validity of the kinetic parameters obtained has been checked comparing the experimental curves with simulated curves.
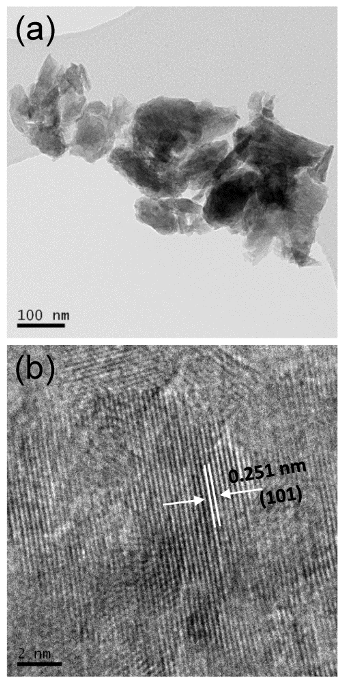
as the synthesis medium evolved from inert atmosphere of argon to hydrogen pressure, the morphology changed from rod like to small particles, with sizes in the range of 25-170 nm.
Constant rate thermal analysis procedure has been applied to the thermal dehydrogenation of MgH2 under high vacuum for the first time.
in high vacuum, the dehydrogenation of MgH2 would take place through instantaneous nucleation in the border line of the crystallites followed by growth of the magnesium particles by diffusion into the bidimensional crystallites.
the dehydrogenation of MgH2 would be explained by assuming that the reaction takes place through a mechanism that implies instantaneous nucleation followed by a growth of nuclei by diffusion in two directions.
From this temperature, the intensity of the MgH2 peaks decreases steadily, while the intensity of the Mg peaks increases in the temperature range 459-567 K.