




Did you find this useful? Give us your feedback























7 citations
6 citations
...The procedure for the CO light-off tests conducted using the SIGU is described by Blades et al.[15] The gas concentrations used were, 10 % CO2, 1 % O2, 0.5 % CO, 5 % H2O and balance N2....
[...]
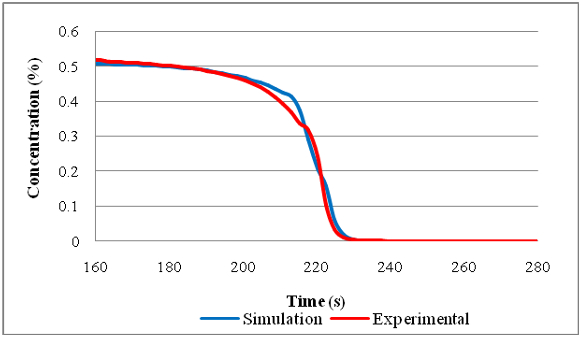
...A study carried out by Blades et al.[15] showed how the QUB global catalyst simulation model, which was first developed by McCullough[16] and later enhanced by Stewart,[17] could accurately simulate the light-off behaviour of catalyst samples for tests conducted on two different laboratory catalyst test rigs....
[...]
...The procedure for the CO light-off tests conducted using the SIGU is described by Blades et al.([15]) The gas concentrations used were, 10 % CO2, 1 % O2, 0....
[...]
...A study carried out by Blades et al.([15]) showed how the QUB global catalyst simulation model, which was first developed by McCullough([16]) and later enhanced by Stewart,([17]) could accurately simulate the light-off behaviour of catalyst samples for tests conducted on two different laboratory catalyst test rigs....
[...]
1 citations
7 citations
...…from the gamma phase, γ-Al2O3, through delta, δ-Al2O3 and theta, θ-Al2O3, to the stable alpha alumina, α-Al2O3, with loss of surface area and hence loss of catalyst activity (Heck et al., 2002; Martinez-Arias et al., 2002; Meyer Fernandes et al., 2010; More et al., 1997; Zanon Zotin et al., 2005)....
[...]
...The washcoat undergoes irreversible phase changes, with the alumina washcoat transforming from the gamma phase, γ-Al2O3, through delta, δ-Al2O3 and theta, θ-Al2O3, to the stable alpha alumina, α-Al2O3, with loss of surface area and hence loss of catalyst activity (Heck et al., 2002; Martinez-Arias et al., 2002; Meyer Fernandes et al., 2010; More et al., 1997; Zanon Zotin et al., 2005)....
[...]
7 citations
...‘surface clean up’ in nitrogen was carried out after the light-off ramps, using a profile of 25°C/min up to 600°C, similar to the pre-treatment procedure suggested by McAtee et al. (2004). Also, at the beginning of each test, the catalyst was pre-treated in nitrogen at 20°C/min up to 300°C, to ‘clean’ the surface....
[...]
5 citations
...Engine ageing followed by laboratory analysis has also been conducted by Usmen et al. (1992), Zhang et al. (1997), Hughes (2005), Kallinen et al. (2005), He et al. (2003) and Hietikko et al. (2004)....
[...]
2 citations
...Engine ageing followed by laboratory analysis has also been conducted by Usmen et al. (1992), Zhang et al. (1997), Hughes (2005), Kallinen et al. (2005), He et al. (2003) and Hietikko et al. (2004)....
[...]