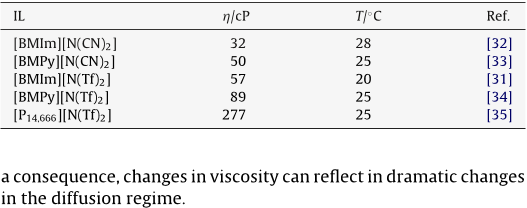
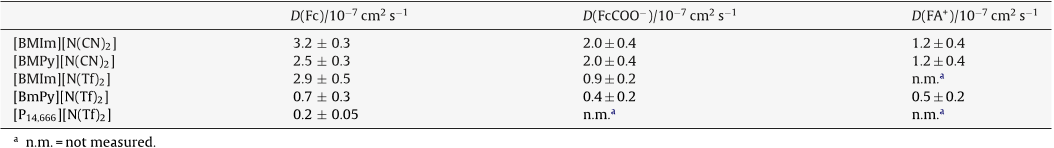
Abstract: The heterogeneous kinetics of the ferrocene/ferrocenium (Fc/Fc+) reaction, as well as the mass transport of Fc and Fc+, were investigated in several bis(trifluoromethylsulfonyl)imide-based ionic liquids, including 1-(1-butyl)-3-methylimidazolium, tri-1-butylmethylammonium, 1-(1-butyl)trimethylammonium, 1-(1-butyl)-1-methylpyrrolidinium, 1-butylpyridinium, and 1-ethyl-3-methylimidazolim bis(trifluoromethylsulfonyl)imide at 303 K. The former investigation was conducted with electrochemical impedance spectroscopy (EIS). The Fc+/Fc reaction was found to be more complicated in these ionic liquids than has been appreciated. EIS measurements indicate that this reaction is electrochemically reversible at freshly activated platinum and glassy carbon electrodes. However, under certain conditions, platinum electrodes develop a surface film that increases the interfacial charge transfer resistance of the Fc/Fc+ reaction, leading to apparent quasireversible behavior. The temperature-normalized diffusion coefficients of Fc and Fc+ scaled linearly with the inverse absolute viscosity in these ionic liquids in accordance with the Stokes-Einstein equation. This expression can be used to calculate the Stokes radii of the diffusing species by the judicious choice of the constant. Reasonable results were obtained for solutes with crystallographic radii greater than those of the ionic liquid constituents when the constant was 6. However, equally good results were obtained for those solutes with radii equal to or slightly less than the radii of the constituent ions, such as Fc and Fc+, when the constant was taken as 4.
![Fig. 5. Cyclic voltammograms recorded at different scan rates at a NEE (geometric area 0.07 cm2; active area 0.004 cm2): (A) 20mM Fc [BMPy][N(Tf)2]; (B) 5mM Fc in [BMIm][N(Tf)2]; (C) 50mM Fc [P14,666][N(Tf)2]. Scan rates: full line 5mV/s; dashed line 50mVs−1; dotted line 500mV/s.](/figures/fig-5-cyclic-voltammograms-recorded-at-different-scan-rates-186vzx3c.png)
![Fig. 6. Dependence of the I/Imax ratio on the scan rate (A) and on the square root of the scan rate (B) for ferrocene oxidation at a NEE in: ( ) [BMPy][N(Tf)2]; (©) [BMIm][N(Tf)2]; ( ) [P14,666][N(Tf)2].](/figures/fig-6-dependence-of-the-i-imax-ratio-on-the-scan-rate-a-and-2eggn6ay.png)
![Fig. 7. (A) Cyclic voltammograms of 1mM FcCOO− in [BMPy][N(Tf2)] recorded a l (](/figures/fig-7-a-cyclic-voltammograms-of-1mm-fccoo-in-bmpy-n-tf2-3kajg4vn.png)

![Fig. 2. (A) Cyclic voltammograms of 1mM Fc in [BMIm][N(CN)2] recorded at GCE (geometric area 0.19 cm2) at different scan rates: 20, 50, 100, 200, 500, 1000mVs−1; peak currents scale with the scan rate. (B) Dependence of the anodic peak current on the square root of the scan rate.](/figures/fig-2-a-cyclic-voltammograms-of-1mm-fc-in-bmim-n-cn-2-19dawvbt.png)
![Fig. 4. Cyclic voltammograms of 1mM Fc in [BMIm][N(CN)2] recorded at an Au-macro electrode at different scan rates: 50, 100, 200 and 500mVs−1; other parameters as in Fig. 3.](/figures/fig-4-cyclic-voltammograms-of-1mm-fc-in-bmim-n-cn-2-recorded-vavr4b8x.png)
![Fig. 3. (A) Cyclic voltammograms of 1mM FcCOOH in [BMIm][N(CN)2] recorded at an Au-macro electrode (geometric area 0.07 cm2), at different scan rates: 10, 20, 50, 100, 200, 400, 800 and 1000mVs−1; peak currents scale with the scan rate. (B) Dependence of the anodic peak current on the scan rate.](/figures/fig-3-a-cyclic-voltammograms-of-1mm-fccooh-in-bmim-n-cn-2-3hvfscoj.png)