Q1. What have the authors contributed in "Iffusion regimes at nanoelectrode ensembles in different ionic liquids" ?
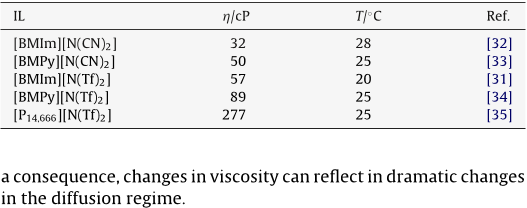
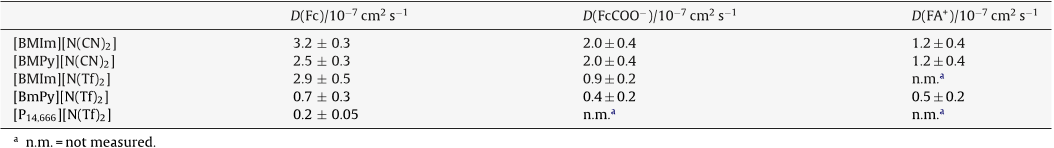
The electrochemical and diffusion behaviour of different redox probes in different ionic liquids is studied at gold nanoelectrode ensembles ( NEEs ) in comparison with millimetre sized gold ( Au-macro ) and glassy carbon ( GC ) disk electrodes. The ILs are the dicyanamide, [ N ( CN ) 2 ] or bis ( trifluoromethylsulfonyl ) amide ), [ N ( Tf ) 2 ] salts of the following cations: 1-butyl-3-methylimidazolium, [ BMIm ], 1-butyl-3-methylpyrrolidonium, [ BMPy ], or tris ( nhexyl ) tetradecylphosphonium [ P14,666 ]. For this reason the diffusion at gold NEEs is studied only in the former ILs.
![Fig. 5. Cyclic voltammograms recorded at different scan rates at a NEE (geometric area 0.07 cm2; active area 0.004 cm2): (A) 20mM Fc [BMPy][N(Tf)2]; (B) 5mM Fc in [BMIm][N(Tf)2]; (C) 50mM Fc [P14,666][N(Tf)2]. Scan rates: full line 5mV/s; dashed line 50mVs−1; dotted line 500mV/s.](/figures/fig-5-cyclic-voltammograms-recorded-at-different-scan-rates-186vzx3c.png)
![Fig. 6. Dependence of the I/Imax ratio on the scan rate (A) and on the square root of the scan rate (B) for ferrocene oxidation at a NEE in: ( ) [BMPy][N(Tf)2]; (©) [BMIm][N(Tf)2]; ( ) [P14,666][N(Tf)2].](/figures/fig-6-dependence-of-the-i-imax-ratio-on-the-scan-rate-a-and-2eggn6ay.png)
![Fig. 7. (A) Cyclic voltammograms of 1mM FcCOO− in [BMPy][N(Tf2)] recorded a l (](/figures/fig-7-a-cyclic-voltammograms-of-1mm-fccoo-in-bmpy-n-tf2-3kajg4vn.png)

![Fig. 2. (A) Cyclic voltammograms of 1mM Fc in [BMIm][N(CN)2] recorded at GCE (geometric area 0.19 cm2) at different scan rates: 20, 50, 100, 200, 500, 1000mVs−1; peak currents scale with the scan rate. (B) Dependence of the anodic peak current on the square root of the scan rate.](/figures/fig-2-a-cyclic-voltammograms-of-1mm-fc-in-bmim-n-cn-2-19dawvbt.png)
![Fig. 4. Cyclic voltammograms of 1mM Fc in [BMIm][N(CN)2] recorded at an Au-macro electrode at different scan rates: 50, 100, 200 and 500mVs−1; other parameters as in Fig. 3.](/figures/fig-4-cyclic-voltammograms-of-1mm-fc-in-bmim-n-cn-2-recorded-vavr4b8x.png)
![Fig. 3. (A) Cyclic voltammograms of 1mM FcCOOH in [BMIm][N(CN)2] recorded at an Au-macro electrode (geometric area 0.07 cm2), at different scan rates: 10, 20, 50, 100, 200, 400, 800 and 1000mVs−1; peak currents scale with the scan rate. (B) Dependence of the anodic peak current on the scan rate.](/figures/fig-3-a-cyclic-voltammograms-of-1mm-fccooh-in-bmim-n-cn-2-3hvfscoj.png)