




Did you find this useful? Give us your feedback














80 citations
28 citations
20 citations
16 citations
15 citations
30 citations
25 citations
24 citations
21 citations
21 citations
The oxygen diffusivity in a matrix with >20 vol% ZrO2 should be very close to that in ZrO2, which leads to a rapid increase in the oxidation rate of SiC particles.
In addition, the formation of ZrSiO4, as a result of the interaction between the matrix and the oxide product, would lead to a reduction of the oxidation rate.
Because the oxygen diffusivity in the matrix now becomes much faster than that in the silica layer around SiC, the oxygen will pass over partially oxidized SiC particles and continuously diffuse into the inner region, leading to the oxidation of more SiC particles and, thus, a deep oxidation zone.
the silica layer of an individual SiC particle in a SiC-containing composite means the layer of SiO2 that is formed as a result of the oxidation reaction occurring on the surface of the SiC particle.
At 1000°C, the diffusivity in ZrO2 is between 10−9 and 10−5 cm2/s; this value is dependent on crystal structure, additives, and the stoichiometry.
Because the oxygen diffusivity slowly increases as the ZrO2 content increases in the range of f < fc, according to the effective medium theory,33,34it is inferred that the oxidation rate will also slowly increase as the ZrO2 content increases.
the oxidation zone of a SiC-containing composite after exposure in an oxidizing environment is defined as the zone from the outermost surface of the composite to the depth where no oxidation of the incorporated SiC particles can be detected.
the substitution of ZrO2 by ZrSiO4 will slow the oxygen diffusion in the matrix, which leads to a decrease in the oxidation rate.
Specimens drawn from the furnace were not reloaded for further oxidation, to avoid the formation of extended cracks due to thermal shock.
percolation theory predicts that Dm has the approximate order of D1 if f < fc and has the approximate order of D2 if f > fc (where D1 and D2 are the diffusivities of a certain species in the two phases, Dm is the diffusivity of that species in the composite, and f is the volume fraction of the second phase).
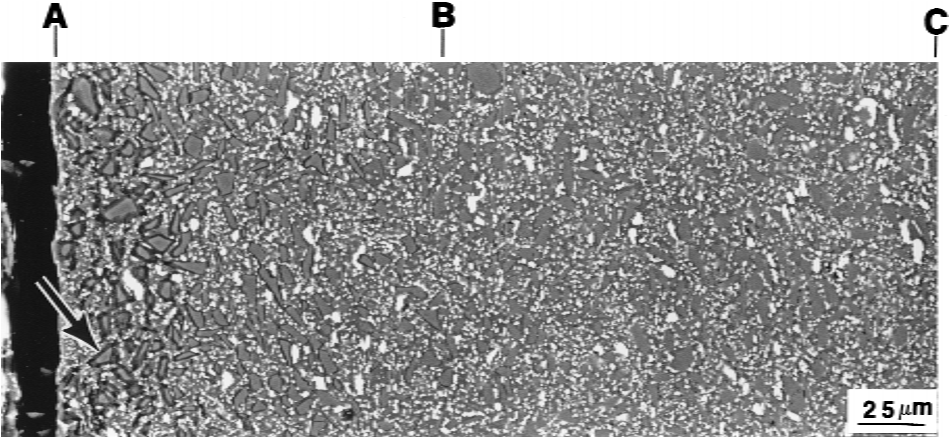
The cross-sectional samples clearly exhibited a distinct layered structure; this layering was due to the different extent of oxidation at various depths, which caused a change in composition.
The SiC particles were slightly oxidized, even at a depth of >600 mm, which indicates a much-larger oxidized depth than that of the MZY15/SiC composite exposed for 500 h.IV.
Because oxygen diffusivity in ZrO2 is much higher than that in mullite (Table II) and using mullite and ZrO2 as the first and second phases, respectively, the relationships of oxygen diffusivity in ZrO2/mullite matrices would beDmatrix O ≈ order of DzirconiaO (if f > fc) (2a)Dmatrix O ≈ order of DmulliteO (if f < fc) (2b)where the superscript denotes the diffusing species (oxygen).
That is, the inward diffusion of oxygen may be controlled by either diffusion through the ZrO2-containing matrix or diffusion through the silica layer, depending on which one is slower.
The fact that the weight gain at 1200°C did not increase (or even slightly decrease) when the ZrO2 content was >80 vol% (Fig. 2) may be attributed to the fact that more ZrSiO4 formed at higher ZrO2 contents, as determined by XRD (Fig. 4).V. Summary
The as-hot-pressed sample consisted of four major phases: mullite, monoclinic ZrO2 (m-ZrO2), tetragonal ZrO2 (t-ZrO2), and SiC (Fig. 3(a)).