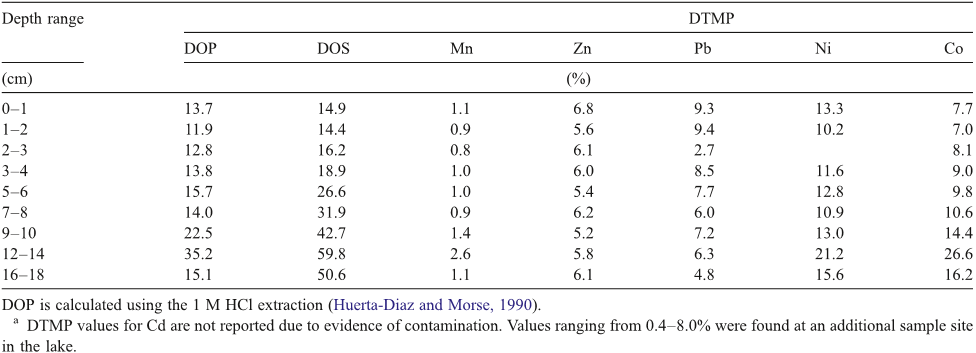
Abstract: Winkler method for dissolved oxygen analysis. Limnol. Oceanog., 10: 135-140. CARRITT, D. E., AND J. H. CARPENTER. 1966. Comparison and evaluation of currently employed modifications of the Winkler method for determining dissolved oxygen in seawater; a NASCO report. J. Marine Res., 24: 286318. CLINE, J. D. 1968. Kinetics of the sulfide-oxygen reaction in seawater; An investigation at constant temperature and salinity. M.S. Thesis, Univ. Washington, Seattle. 68 p. CUSTER, J. J., AND S. NATELSO?\T. 1949. Spectrophotometric determination of microquantities of iodine. Anal. Chem., 21: 1005-1009. THOMPSON, T. G., AND R. J. ROBINSON. 1939. Notes on the determination of dissolved oxygen in sea water. J. Marine Res., 2: 1-8. WHEATLAND, A. B., AND L. J. SMITH. 1955. Gasometric determination of dissolved oxygen in pure and saline water as a check of titrimetric methods. J. Appl. Chem. (London), 5: 144-148. WINKLER, L. W. 1888. Die Bestimmung des im Wasser gelosten Sauerstoffes. Chem. Ber., 21: 2843-2855.