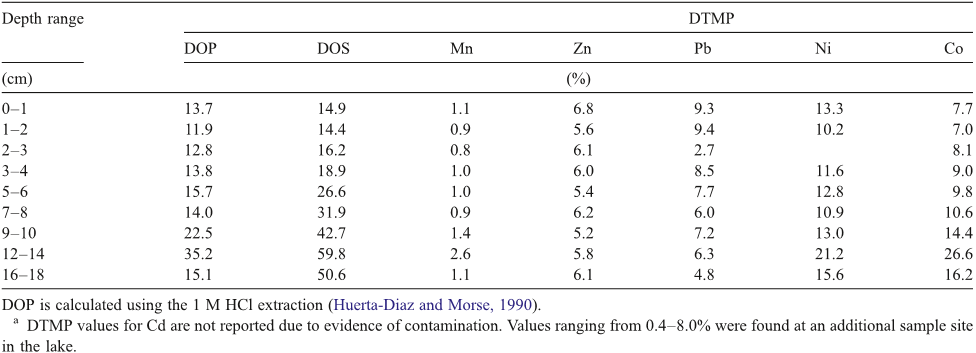
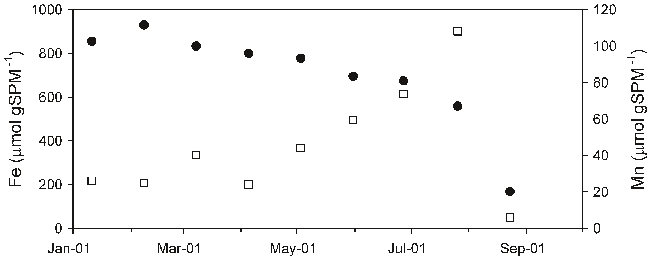
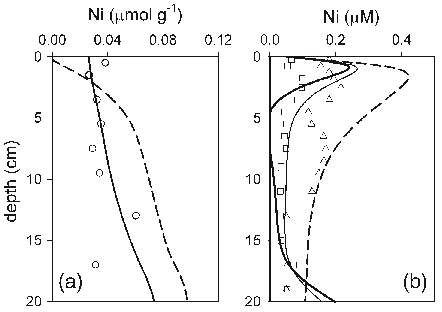
Abstract: The concentrations of trace metals in sewage water and sludge samples from River Kubanni drainage basin in Zaria City, Nigeria were investigated in this study. The drainage basin is utilized as a source for irrigation water, during dry seasons. The sewage water quality characteristics in three month sampling periods, that is, February - April, 2008 (peak of dry season and period of intensive usage of the sewage water), the speciation of metals in the sewage sludge from the drainage basin, and the risk to sewage water column contamination were evaluated. The sewage water quality characteristics were mostly beyond the recommended irrigation water standards by the food and agriculture organization (FAO) and United State environmental protection agency (USEPA) except for zinc and nickel. In addition, the average values of Cd, Cu, Pb, Cl - and NO 3 - in sewage water samples analyzed were higher than the respective reference values for irrigation water. To study the speciation of metals in sewage sludge, five metals (Zn, Ni, Cu, Pb and Cd) in the sludge were subjected to sequential extractions. The metals analyzed were distributed in both the non-residual and residual phases. Total extractable trace metals in sewage sludge were: Zn (403.3 mg/kg dry weight), Ni (184.2 mg/kg dry weight), Cu (303.4 mg/kg dry weight), Pb (129.0 mg/kg dry weight) and Cd (19.7 mg/kg dry weight). However, there was low risk to sewage water contamination based on the calculated individual contamination factors (ICF) obtained for sewage sludge from the trace metal sequential extractions. From the calculated individual contamination factors, Ni and Zn followed by Cd and Pb posed the highest risk to sewage water contamination. Based on this study, the human health is at risk, since sewage water from the drainage basin has been the source for irrigation water during dry seasons, which might lead to trace metal ingestion by soil and subsequently by vegetables. Thus, this might become important pathways of human exposure to metal contamination. Key words: Trace metals, speciation, contamination factor, sewage water and sludge.