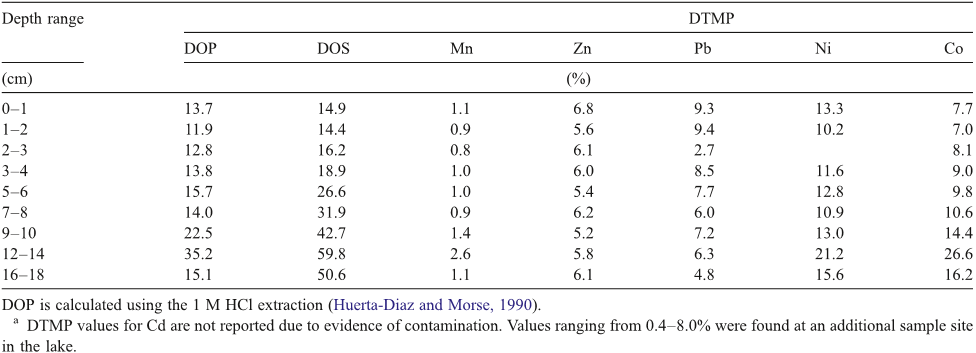
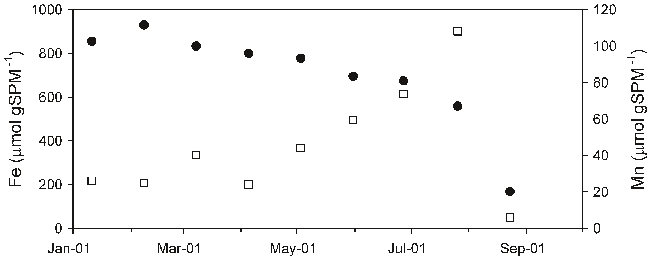
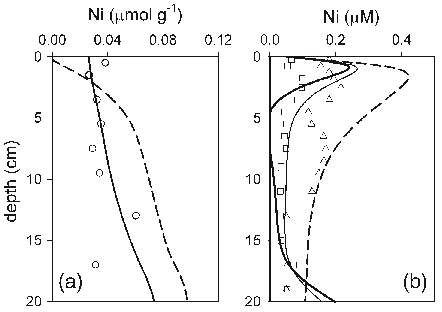
Abstract: L’etude realisee porte sur l’evaluation du niveau de contamination par les elements traces metalliques (ETM) des sediments de la rade de Toulon, une rade semi-fermee soumise a un fort impact anthropique. Le prelevement de carottes d’interface en 52 points repartis sur l’integralite de la Rade a permis d’etablir une cartographie precise des caracteristiques sedimentaires et des teneurs en metaux/metalloides. Les resultats obtenus sur les sediments de surface ont montre l’etat de contamination significatif de la rade (en particulier en Cu, Hg, Pb, et Zn), notamment dans les zones les plus enclavees de la petite rade, ou les teneurs peuvent depasser de plusieurs ordres de grandeur les limites definies par la legislation en vue d’operations de dragage. La distribution de la contamination observee a clairement indique un export de la petite vers la grande rade (normalement moins exposee), probablement gouverne par des processus hydrodynamiques responsables de la remise en suspension du sediment contamine. Les profils sedimentaires de carottes d’interface prelevees dans des zones de contamination contrastee ont revele la presence systematique de pics de contamination dans les 20 premiers cm. Compte tenu des taux de sedimentation determines, ceci demontrerait que la rade a ete soumise a un episode de multi-contaminations majeur, probablement lie aux consequences de la 2nde guerre mondiale. L’analyse des eaux interstitielles et surnageantes (parametres physico-chimiques, majeurs, traceurs diagenetiques et ETM) de ces carottes d’interface a permis d’etudier la mobilite des ETM dans le sediment. Les profils obtenus apparaissent essentiellement controles par des mecanismes diagenetiques et demontrent le role exerce par les principales phases porteuses presentes dans le sediment (oxy-hydroxydes de Fe et de Mn, sulfures) sur la mobilite des ETM. La modelisation de ces profils a permis d’evaluer les flux diffusifs a l’interface eau sediment, afin de determiner l’action du sediment, en tant que puits ou source de contamination pour la colonne d’eau. Les flux diffusifs sortant calcules apparaissent relativement faibles en comparaison des teneurs totales mesurees dans le sediment, demontrant que la majorite des ETM est fortement immobilisee dans le sediment.Enfin, ce travail a ete complete par des experiences de remise en suspension en laboratoire et sur le terrain, visant a simuler differents scenarios possibles (tempete,trafic maritime, dragage). Dans les conditions etudiees, si pour certains ETM la remobilisation en solution est faible (ex. As, Hg), elle peut au contraire etre tres significative pour d’autres (ex. Cd, Cu, Pb) conduisant a une contamination non negligeable de la colonne d’eau.