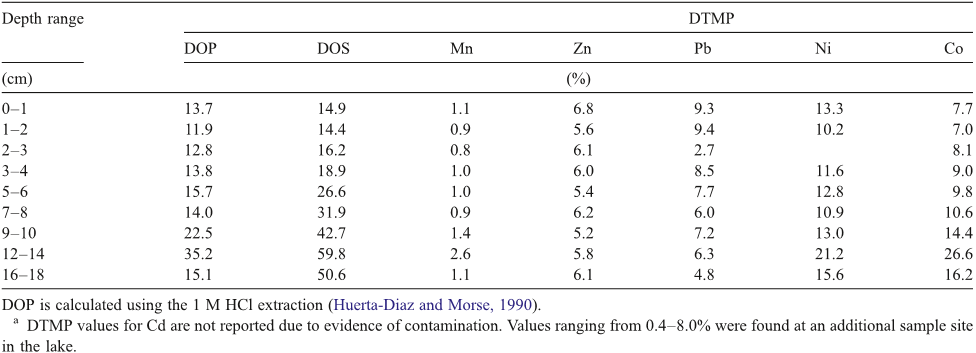
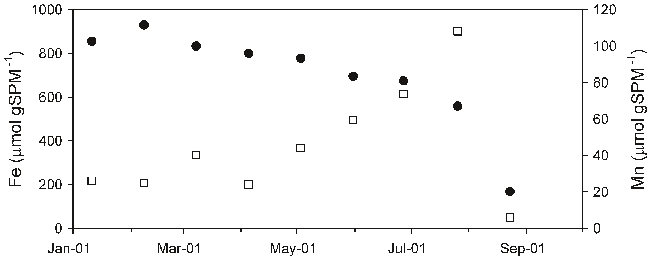
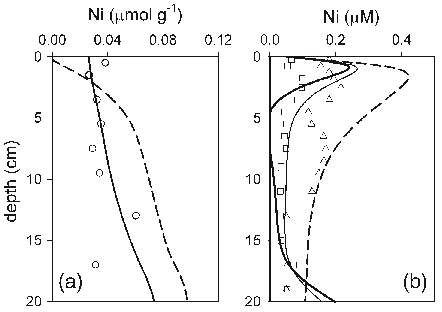
Abstract: Changes in hydrology, and developments in morphology, water quality, and ecology of the Rhine-Meuse estuary after its enclosure in 1970 are analyzed on the basis of existing monitoring data. Annual averages of ammonium, nitrate plus nitrite, total phosphate, total lead, and chlorophyll-a concentrations as well as transparency of the water are presented. Abundances of some water bird species are given for the period 1970–1993, and the relative fish biomass for the period 1971–1988 is discussed. The gradual evolution led toward the dominance of bream. The area has developed toward a system with generally low natural ecological values. Ecological impacts of present sluice management are discussed and include the accumulation of contaminated sediments, disappearance of intertidal areas and nursery grounds for fish, disturbance of fish migration, and less mixing of river and seawater. Recent policy developments have brought the present management of the Haringvliet sluices back in to discussion. A recent policy document has presented several management alternatives, including partial and complete reopening of the sluices to permit saltwater intrusion. Three management options are compared in terms of costs and ecological benefits. It is concluded that a complete reopening, and thus a partial restoration of the estuarine characteristics, is most beneficial for the ecosystems of the area itselt, for the upstream Rhine and Meuse rivers, and for the adjacent North Sea, but costs amount to about 600 million US $.