




Did you find this useful? Give us your feedback


![Fig. 1. Risk maps obtained using weights k1 = 0.1, k2 = 0.9. The computed risk values are normalized to the range of [0, 1]. Blue, white and red regions represent regions of low, medium and high risk, respectively. Black regions depict impermissible insertion areas. (a) Interactive planning by moving the target point within a region. Our high performance approach opens the possibility of interactive intra-operative visualization for computer assisted re-planning. (b) Moving the target point along a selected path. The shown risk maps are computed by placing the current target at the tip of the needle. The horizontal bar shows the normalized risk as a function of the percentage of covered pathway (i.e. 50% means that the needle is currently at half of the total length of the selected safe path). The insertion velocity can be planned according to the risk function along the selected path.](/figures/fig-1-risk-maps-obtained-using-weights-k1-0-1-k2-0-9-the-32cgnmr6.png)
![Fig. 7. Comparison between voxel-based approach [3] versus our mesh-based approach in terms of computational time. The equivalence of input structures is as follows: R1 is a 32k Verts-65k Tris mesh (for our meshbased approach), or a 288x172x136 grid (for the voxelbased approach); R2 is a 62k Verts-126k Tris mesh, or a 576x172x136 grid; R3 is a 124k Verts-248k Tris mesh, or a 576x460x136 grid; R4 is a 185k Verts-370k Tris mesh, or a 576x768x136 grid; R5 is a 246k Verts-492k Tris mesh, or a 576x768x272 grid.](/figures/fig-7-comparison-between-voxel-based-approach-3-versus-our-2u60a9ma.png)










34 citations
28 citations
21 citations
...Currently the algorithms are written in C++, incorporating appropriate image processing libraries (such as ITK), and optimizing it with multithread implementation [13] and GPU acceleration [14]....
[...]
21 citations
...The hardware/GPU acceleration and/or software-based preprocessing of the data to facilitate interactive selection of trajectory are described in [13,18,19]....
[...]
19 citations
...In [26], the estimated risk associated to accessing path was visualized interactively thanks to GPU optimized methods....
[...]
413 citations
334 citations
107 citations
...Index Terms—GPU Acceleration, Neurosurgical Interventions, Risk Maps, and Straight Access...
[...]
In the future, the authors plan to further investigate efficient approaches for procedures on dynamically changing structures, secondary to breathing, cardiac beating, and/or to the interventional procedure. Another future research direction would be to extend the current work for efficient planning and visualization of surgical interventions with non-straight access paths.
Integration between the planning assistance software, the imaging acquisition mechanisms, and the surgical plan execution tool is the key in the intra-operative scenario.
With respect to the problem of computing the risk map, a naive scheme of assigning work to processing units in CUDA or OpenCL would correspond to launch the execution of a grid with as many threads as the paths.
Since the closest vertex is closer to their path than the closest point in bounding box AABB2,2, the authors can safely discard traversals through such node.
Although the computational time of both the mesh-based and voxel-based approaches is approximately linear to the number of computed paths, the voxel-based approach has the additional disadvantage of growing linearly with the size of the input structure.
Their current clinical workflow for using the herein proposed approach entails interactively modifying (with a pointing device such as a mouse) the location of the target point and visualizing the effects on the risk maps.
A common practice is manual selection and assessment of the suitability of different entrance positions on the scalp surface of the patient in order to select an appropriate insertion path.
The possibility of performing thisprocess in real-time or near real-time allows interactive visualization and planning, which would significantly reduce the planning time and thus allow effective intraoperative re-planning of neurosurgical interventions if needed.
Testing each path for intersection against each triangle, and computing its closest distance to each vertex are not affordable options for interactive visualization and planning.
An efficient GPU implementation of their approach further enables the computation of risk maps at an interactive rate even for high-resolution meshes.
When the operator selects a path, the path is usually further analyzed and inspected by looking at imaging planes (MR slices) orthogonal to the insertion path (also known as bird’seye view).
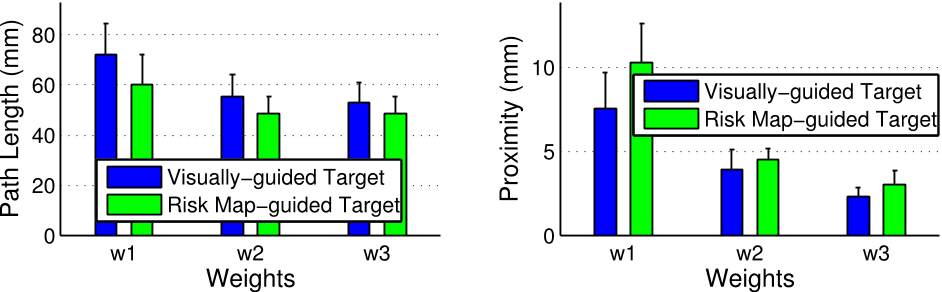
The authors define such a function based on the following criteria: (1) the paths cannot intersect any critical tissue, otherwise, this may result in unacceptable patient injury; (2) the paths should be as distant as possible from the critical tissue; and (3) the path length (i.e. the distance between the entry point and the target) should be as short as possible.
the workload assigned to some blocks will need moretime than other blocks, eventually leading to a situation where some of the SMs are idle for a portion of the overall computation time.
In their work, long-running warps are those that only finish their execution when all the paths in the global queue have been processed.