Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes
Summary (2 min read)
1. Introduction
- Iron nanoparticles were readily synthesized using green tea leaf extracts (GT-Fe NPs).
- Green tea is known to contain polyphenols that act both as a reducing agent and a capping agent.
- The main objective of the current study is to test the applicability of GT-Fe NPs as a Fenton catalyst in the removal of cationic (methylene blue) and anionic (methyl orange) model dyes.
- Another objective of the study is to compare the extent of degradation of GT-Fe NPs with that of nZVI produced by borohydride reduction (BH-Fe NPs).
- At room temperature it appears as a solid, odorless, dark green powder, which yields a blue solution when dissolved in water.
2.1. Preparation of GT-Fe NPs
- The synthesis of iron nanoparticles using green tea extracts was described previously [17, 18] .
- The green tea extract was prepared by heating 60.0 g L −1 green tea (Alwald Brand) until boiling.
- After settling for 1.0 h, the extract was vacuum-filtered.
- Following this, 1.0 M NaOH solution was added until the pH was 6.0 and the formation of GT-Fe NPs was marked by the appearance of intense black precipitate.
- The iron particles were then separated first by evaporating water from the iron solution on a hot plate (Freed Electric), and then by drying it overnight in a fume hood.
2.4. Characterization of GT-Fe NPs
- For XPS, samples were analyzed under high vacuum using a Thermo Fisher Scientific Escascope X-ray photoelectron spectrometer equipped with a dual anode (Mg/Al) source and a concentric hemispherical electron energy analyzer.
- Data acquisition and manipulation were carried out using Pisces (Dayta Systems, UK) software.
- Charge referencing was carried out against adventitious hydrocarbon (C 1s = 284.8 eV binding energy).
- Nanoparticle samples were sonicated in analytical grade methanol for 30 s and mounted on 200 mesh holey carbon coated copper grids.
3.1. Characterization of GT-Fe NPs
- The same elements appearing in the EDX spectrum are also observed using XPS spectra.
- Auger electron peaks are observed for Na and O in addition to their photoelectron peaks.
- These signals are thought to arise primarily from the C atoms in the polyphenol groups attached to GT-Fe NPs, with the duplet indicating two types of C with different chemical environments.
- These peaks are attributed to the polyphenols presumably present at the surface of iron nanoparticles.
3.2.1. Fenton-like mechanism and pH effect
- Eqs. (i) and (ii) show that ferrous ions initiate the reaction leading to production of hydroxyl radicals, which then attack the organic pollutants leading to their degradation.
- Iron oxides, iron oxohydroxide, and zero valent iron can be used as a source of ferrous ions in a Fenton-like process.
- In the case of azo dyes, the cleavage of the azo bond (-N N-) in the chromophore of the dye leads to decolorization of the dye solution [20] .
- The surface area and surface activity of the Fenton catalyst are crucial.
- Doubtlessly, the nanosize of the GT-Fe material greatly enhances its activity as a catalyst.
3.2.3. Kinetic experiments
- Eqs. ( 3) and ( 4) were employed to describe the data corresponding to the pre-equilibrium stage (up to 120 min) for both dyes.
- The results of the linear regression analysis were used to calculate the rate constants for each case.
- At a given liquid concentration of a solute, for a fixed rate constant, the variation of the rate is more pronounced in the case of second order kinetics compared with first order.
- For a given rate order (first or second), the response of the rate at a given concentration will depend on the value of the rate constant.
- In both cases, the larger rate constant of MB reflects its faster removal kinetics.
3.2.4. Effect of dye concentration
- To further assess the efficiency of GT-Fe NPs as a catalyst, blank experiments were performed without the addition of the material to the peroxide containing reaction mixture.
- The results showed less than 3% removal of the dye even in the most dilute solution.
3.3. Comparison of GT-Fe NPs with BH-Fe NPs
- Nevertheless, the reported results must not undermine the effectiveness of BH-Fe NPs in dye removal.
- Preliminary data of their ongoing experiments suggest that BH-Fe NPs would be much more effective than GT-Fe NPs when used directly (not as a Fenton-like catalyst; without H 2 O 2 ) to catalyze dye degradation/sorption.
4. Conclusions
- As a Fenton-like catalyst, GT-Fe NPs demonstrated impressive removal capabilities towards both of MB and MO, in terms of the extent of removal and the kinetics.
- Compared with BH-Fe NPs, GT-Fe NPs showed higher dye removal percentages and faster kinetics when used as a Fenton-like catalyst.
Did you find this useful? Give us your feedback
Figures (12)

Table 1 Rate constants obtained from linear regression analysis of the data of dye removal. 
Fig. 7. Linear plots of the kinetic data: (a) first order plot for MB, (b) secon 
Table 2 The percentage removal of MB and MO at different initial concentrations when GT-Fe NPs are used as a Fenton-like catalyst. Cl stands for the equilibrium liquid concentration. 
Fig. 8. (a) SEM image, and (b) EDX spectrum of BH-Fe NPs. 
Fig. 3. A typical XRD pattern of GT-Fe NPs. 
Fig. 2. (a and b) TEM images, and (c) EDX spectrum, of GT-Fe NPs. 
Table 3 Kinetic and equilibrium data corresponding to removal of MB when borohydride-reduced Fe (BH-Fe NPs) is used as a Fenton-like catalyst. 
Table 4 Kinetic and equilibrium data corresponding to removal of MO when borohydride-reduced Fe (BH-Fe NPs) is used as a Fenton-like catalyst. 
Fig. 9. XRD pattern of BH-Fe NPs. 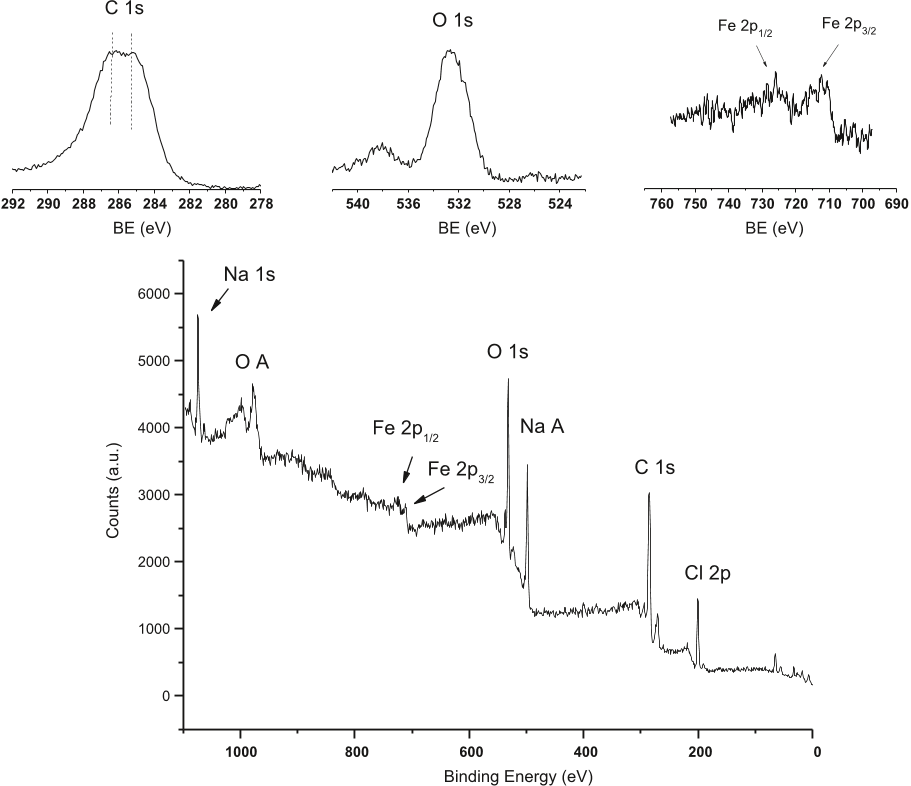
Fig. 4. XPS spectra of GT-Fe NPs. The insets sh 
Fig. 5. FTIR bands corresponding to: (a) polyphenols, (b) MB, and (c) MO. The 
Fig. 6. Variation of dye concentration with time: (a) MB, and (b) MO. The inset
Citations
1,549 citations
778 citations
479 citations
Cites background from "Green synthesis of iron nanoparticl..."
...It is reported that the heterogeneous MFe2O4 is extensively used as catalyst due to its high chemical and thermal stability (Kurian and Nair, 2015) Magnetically separable nano-particles of iron oxide can be used as Fenton catalysts for removal of several types of pollutants (Shahwan et al., 2011; Sun and Lemley, 2011)....
[...]
464 citations
Cites background from "Green synthesis of iron nanoparticl..."
...performed photodegradatio of methyl blue and methyl orange [13], a d f und that the pH of solution and steric structure were highly related to photocatalytic fficiency....
[...]
...performed photodegradation of methyl blue and methyl orange [13], and found that the pH of solution and steric structure were highly related to photocatalytic efficiency....
[...]
...This phenomenon was studied for other dye molecules, including the AR14/TiO2 [3] and other systems [13,14,68,69]....
[...]
...During the photodegradation reaction, a redshift or blueshift can be sometimes seen in the characteristic absorption of dye molecules, possibly caused by the aggregation of organic dyes [13,30]....
[...]
389 citations
References
11,753 citations
871 citations
668 citations
565 citations