




Did you find this useful? Give us your feedback













48 citations
47 citations
26 citations
26 citations
...Rheology regards the study of the material flow and deformation behaviour and may be measured by applying an external force (shear-induced deformation) to a sample [3]....
[...]
13 citations
100 citations
...The application of HME for manufacture of pharmaceuticals has been widely reported including pellets [3], sustained release tablets [4,5] implants [6], and transdermal films [7]....
[...]
97 citations
...The rule has been studied in detail [34] and found to hold for most polymer melts, although it may not hold for more complex binary and tertiary mixtures or for polymers which exhibit thermal sensitivity....
[...]
84 citations
80 citations
...(FVM) [12], and smoothed particle hydrodynamics (SPH) [13]....
[...]
...Recent progress in HME simulation has been made using finite element method (FEM) [11], finite volume method (FVM) [12], and smoothed particle hydrodynamics (SPH) [13]....
[...]
Increasing temperature provides the polymer molecules with greater mobility allowing them to flow more freely whereas plasticizers act to reduce the internal resistance of the polymer melt by effectively lubricating the flow of polymeric chains.
Motor torque and material throughput can also be recorded and used to give an indication of specific energy input to the melt, which is related to the temperature, material properties and degree of filling in the extruder screw channels.
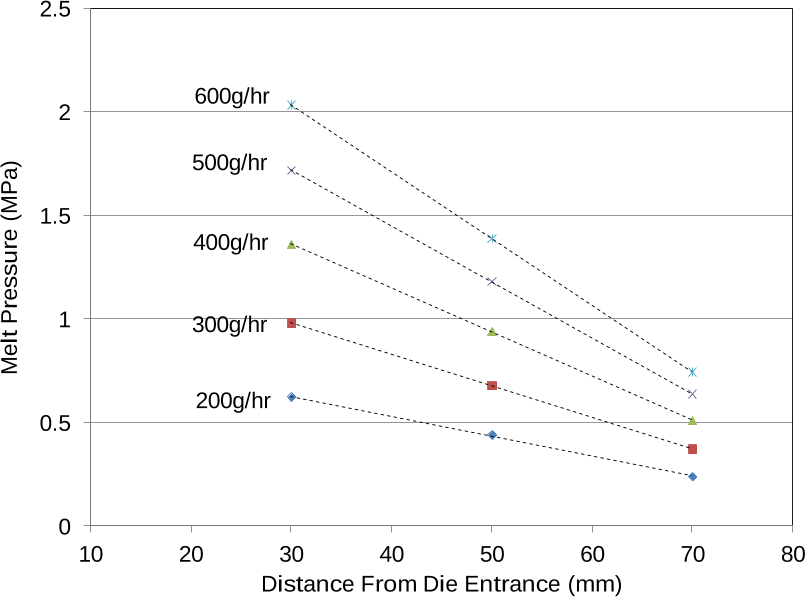
At each set throughput, the process was allowed a 15 minute stabilization period before pressure measurements were recorded, for a representative period of around 10 minutes.
In-line rheological slit dies have also been used in twin screw extrusion, both for monitoring reactive extrusion processes such as cross-linking of polyethylene 29 and for incorporation of additives such as the flame retardant magnesium hydroxide into a polyethylene 30 .
Three pressure transducers (Dynisco PT435) with a full scale deflection of 10.3 MPa were flush mounted at the surface of the slit and monitored at a frequency of 1
Real-time assessment of rheology within the extrusion process can be achieved by measurement of pressure drop inside an instrumented extruder die.
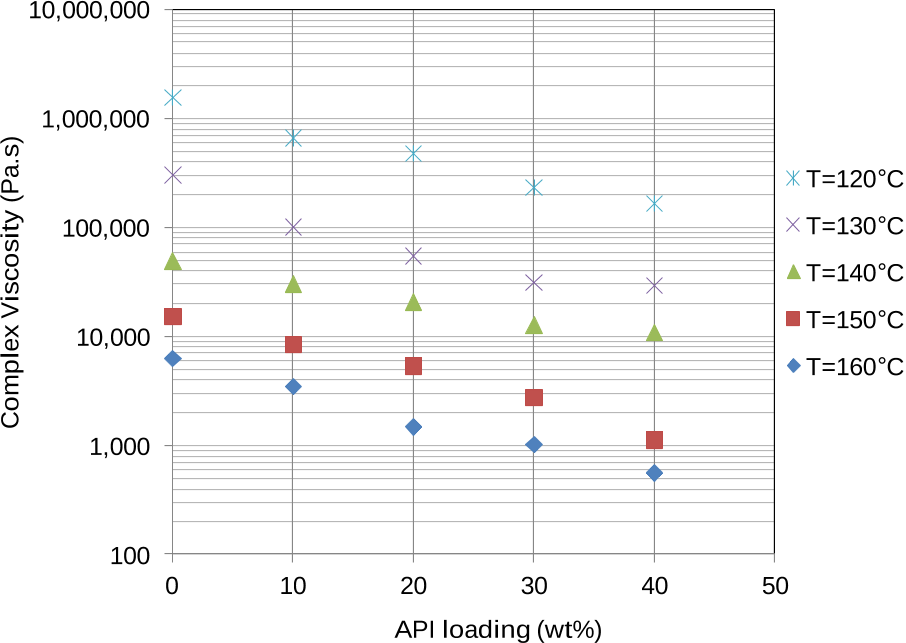
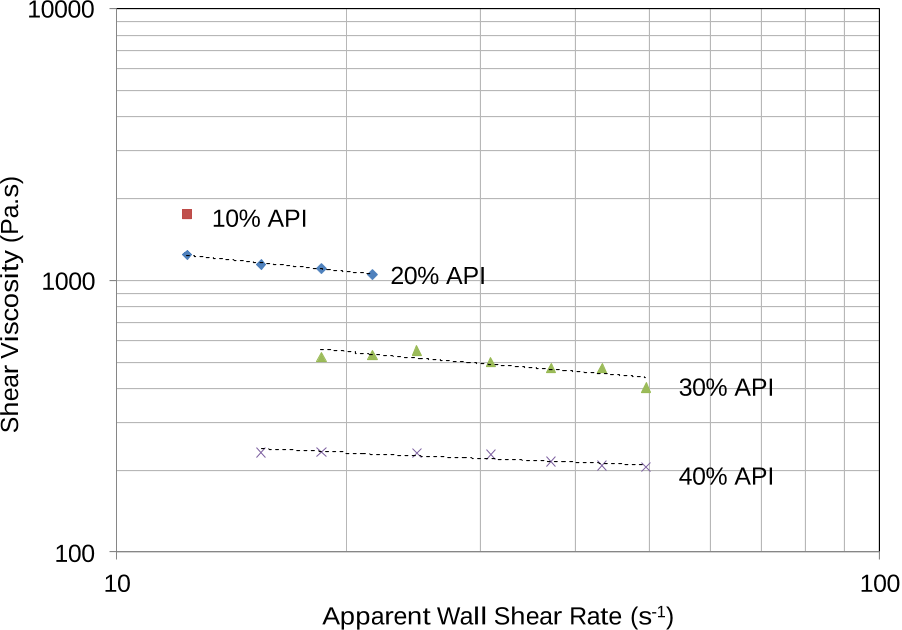
Results showed that the compound displayed shear thinning behavior in the mixtures tested and that shear viscosity decreased with increase in API loading, reflecting miscibility of the API within the polymer matrix.
The plasticization effect of the API was also clearly observed, with shear viscosity decreasing from 1150 Pa.s at 20 % w/w API to 233 Pa.s at 40 % w/w API at an apparent shear strain rate of 15 s -1 .
Such measurements can be used as a quality control indicator, using statistical process control and trending analysis to detect deviations from the desired set point.
Rheology has been used to characterize pharmaceutical solid dispersions and solutions, although only a relatively small number of studies have been reported.
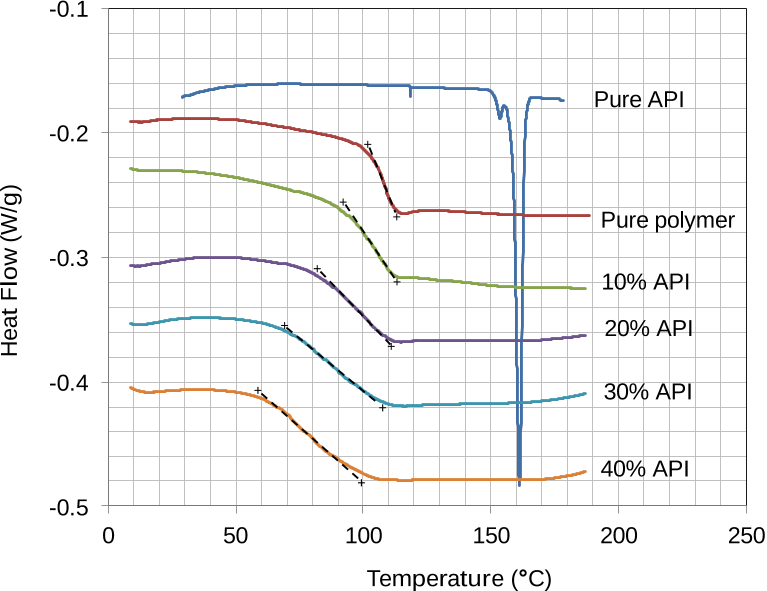
It appears likely that this exposure to high temperature and mixing had an effect on the consistency of the flow properties of the compound, increasing the level of plasticization and mixing.
This indicated that the API was readily miscible within the polymer matrix in the molten state, and that the API had a plasticising effect.