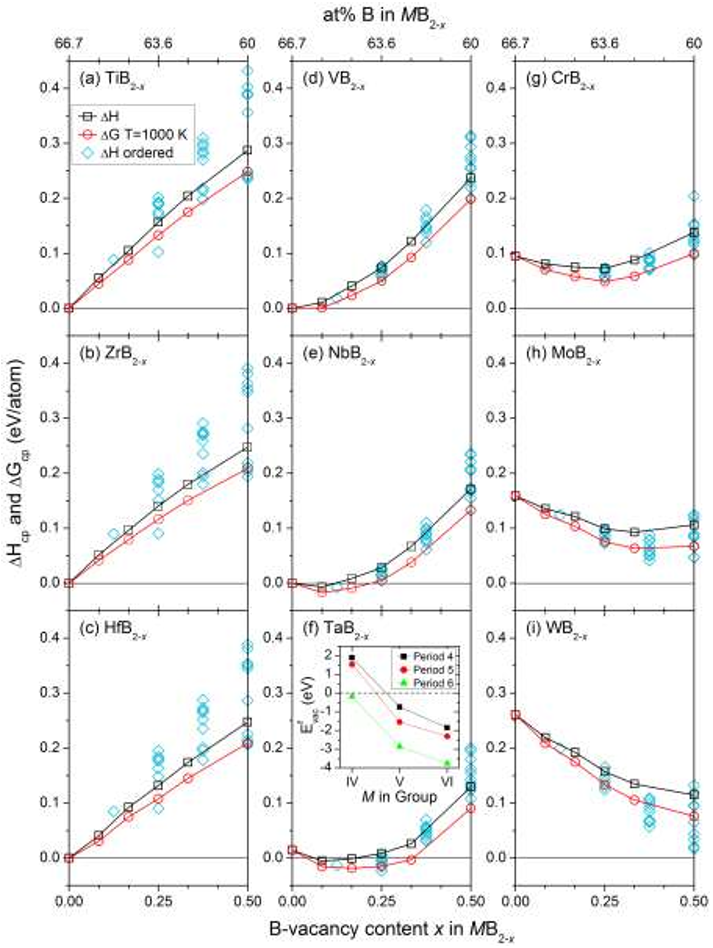
Influence of boron vacancies on phase stability,
bonding and structure of MB2 (M = Ti, Zr, Hf,
V, Nb, Ta, Cr, Mo, W) with AlB2 type structure
Martin Dahlqvist, Ulf Jansson and Johanna Rosén
Linköping University Post Print
N.B.: When citing this work, cite the original article.
Original Publication:
Martin Dahlqvist, Ulf Jansson and Johanna Rosén, Influence of boron vacancies on phase
stability, bonding and structure of MB2 (M = Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, W) with AlB2
type structure, 2015, Journal of Physics: Condensed Matter, (27), 43, 435702.
http://dx.doi.org/10.1088/0953-8984/27/43/435702
Copyright: IOP Publishing: Hybrid Open Access
http://www.iop.org/
Postprint available at: Linköping University Electronic Press
http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-122410

1
Influence of boron vacancies on phase stability, bonding and
structure of MB
2
(M = Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, W) with
AlB
2
type structure
Martin Dahlqvist
1*
, Ulf Jansson
2
, Johanna Rosen
1
1
Thin Film Physics Division, Department of Physics, Chemistry and Biology
(IFM), Linköping University, SE-581 83 Linköping, Sweden.
2
Department of Chemistry, The Ångström Laboratory, Uppsala University,
Uppsala SE-751 21, Sweden
* Electronic mail: madah@ifm.liu.se
Transition metal diborides in hexagonal AlB
2
type structure typically form stable MB
2
phases for group IV elements (M = Ti, Zr, Hf). For group V (M = V, Nb, Ta) and group
VI (M = Cr, Mo, W) the stability is reduced and an alternative rhombohedral MB
2
structure becomes more stable. In this work we investigate the effect of vacancies on
the B-site in hexagonal MB
2
and its influence on the phase stability and the structure for
TiB
2
, ZrB
2
, HfB
2
, VB
2
, NbB
2
, TaB
2
, CrB
2
, MoB
2
, and WB
2
using first-principles
calculations. Selected phases are also analyzed with respect to electronic and bonding
properties. We identify trends showing that MB
2
with M from group V and IV are
stabilized when introducing B-vacancies, consistent with a decrease in the number of
states at the Fermi level and by strengthening of the B-M interaction. The stabilization
upon vacancy formation also increases when going from M in period 4 to period 6. For
TiB
2
, ZrB
2
, and HfB
2
, introduction of B-vacancies have a destabilizing effect due to
occupation of B-B antibonding orbitals close to the Fermi level and an increase in states
at the Fermi level.

2
1. Introduction
Transition metal borides exhibit an interesting combination of properties such as high hardness,
low wear rate and excellent electrical conductivity, making them suitable for various thin film
applications. A large number of boride phases with different crystal structures are known. One
of the most common structure types is the hexagonal AlB
2
structure (P
6
/mmm) typically formed
by metal (M) constituents from groups IV through VI. This structure can be described as close-
packed layers of the metal separated by planar layers of boron. The boron atoms form a
honeycomb network with strong B-B bonds within the layer. The stability of the AlB
2
structure
is dependent on the transition metal M. Typically, the group IV elements (Ti, Zr, Hf) form stable
MB
2
phases with a limited homogeneity range. Going to group V (V, Nb, Ta) and group VI
(Cr, Mo, W), the stability of the hexagonal MB
2
is reduced, and an alternative rhombohedral
MB
2
structure (R
m) with a puckered boron layer becomes more stable for transition metals in
group VII and VIII. The reduced stability of the hexagonal MB
2
structure can be explained by
trends in the electron structure. Analysis of the density of states (DOS) from density functional
theory (DFT) calculations show a pseudogap separating bonding and antibonding M-d/B-p
states [1, 2]. For the group IV transition metals, the Fermi level is positioned in the gap, filling
all the bonding states. For the group V and VI elements, antibonding states are also filled,
leading to a reduced stability of the structure. The stability can be affected by vacancies and
other point defects. For example, it is well-known that the NbB
2
phase exhibits a homogeneity
range of 65-70 at% B corresponding to a composition NbB
1.84
to NbB
2.34
[3]. Most likely, these
vacancies are formed on both metallic and boron sites. Other metal diborides, however, such as
CrB
2
exhibit no homogeneity range from the published phase diagrams [4].
Recently, we have observed that thin film synthesis from MB
2
(M = Nb, Cr, Mo) targets with a
clear boron deficiency (B/M < 2) resulted in thin films exhibiting a B/M ratio ranging from 1.5
to 1.8 [5-7]. For the Cr-B and Mo-B systems, these compositions should lead to a mixture of
phases including also more complex structures such as Cr
3
B
4
and MoB. However, the phase
analysis only showed the formation of substoichiometric hexagonal NbB
2-x
, CrB
2-x
, and MoB
2-
x
. The possibility to deposit highly substoichiometric MB
2
films by magnetron sputtering raises
a number of questions regarding their stability and the effect of vacancies on the materials
properties. No systematic study has yet been carried out to study the effect of vacancies on the
AlB
2
-type borides for the early transition metals. The aim with this work is therefore to use
first-principles calculations to investigate how B-vacancy formation in hexagonal MB
2
(M = Ti,
Zr, Hf, V, Nb, Ta, Cr, Mo, W) affects the stability and structure, and for selected compositions

3
also electronic structure. A comparison of MB
2
phases with M from group IV (M = Ti, Zr, Hf),
V (M = V, Nb, Ta), and VI (M = Cr, Mo, W) will illustrate trends in vacancy formation for
increasing number of d-electrons, while a comparison of M from period 4 (M = Ti, V, Cr), 5 (M
= Zr, Nb, Mo), and 6 (M = Hf, Ta, W)will show the trends in vacancy formation going from a
3d to a 4d to a 5d metal.
2. Computational details
B-vacancies in MB
2
are modeled with the special quasi-random structure (SQS) method [8] to
mimic an ideal random alloys of B-vacancies on the B-sites. SQS supercells were generated
from 4×4×3 unit cells of the AlB
2
-prototype structure at various B-vacancy concentrations x by
optimizing the Warren-Cowley pair short-range order parameters [9, 10] up to the 8
th
shell. In
total there are 48 M-sites at Wyckoff site 1a and 96 B-sites at Wyckoff site 2d, and Table I
summarizes information for the supercells used to model MB
2-x
. Figure 1(a) shows a supercell
of MB
2-x
with x = 0.167. The size of the supercell, 4×4×3 unit cells, is needed to obtain
converged energies and bulk modulus while smaller supercells only give qualitative accurate
equilibrium volumes. In addition, different ordered B-vacancy structures using a 2×2×2 unit
cells with 23 (x = 0.125), 22 (x = 0.25), 21 (x = 0.375), and 20 (x = 0.5) atoms per cell were
considered. These are defined in Table II along with enumerated B atoms in Fig. 1(b).
Table 1. Data for MB
2-x
supercell of size 4×4×3 unit cells (4a × 4a × 3c).
x
at% B
# M atoms
# B atoms
# total atoms
0.000
66.7
48
96
144
0.083
65.7
48
92
140
0.167
64.7
48
88
136
0.250
63.6
48
84
132
0.333
62.5
48
80
128
0.500
60.0
48
72
120

4
Figure 1. Schematic illustration of (a) a 4×4×3 SQS supercell used for modeling MB
2-x
where
x = 0.167, and (b) a 2×2×2 supercell, with enumerated B atoms, used for modeling ordered B-
vacancies for x = 0.25 and 0.50. Red atoms represent B-vacancies, and M and B atoms are
shown in black and green, respectively.
Table 2. List of ordered B-vacancies considered for MB
2-x
where x = 0.125, 0.25, 0.375, and
0.5. The position of the B-vacancies are given by the numbers within parenthesis, which
correspond to the enumeration of B-atoms in Fig. 1(b).
x
B-vacancy enumeration
0.125
(1)
‡
0.25
(1, 2)
‡
, (1, 3), (1, 4), (1, 7), (1, 8), (1, 9)
‡
, (1, 10)
0.375
(1, 2, 8), (1, 2, 9), (1, 2, 10), (1, 3, 8), (1, 3, 9), (1, 3, 10), (1, 3, 11)
‡
,
(1, 4, 7), (1, 4, 8)
0.5
(1, 2, 7, 8), (1, 2, 8, 9), (1, 2, 9, 10), (1, 2, 10, 11), (1, 3, 7, 9),
(1, 3, 8, 10), (1, 3, 9, 11), (1, 3, 10, 12), (1, 4, 7, 10), (1, 4, 8, 11)
‡
Ordered structures used in Ref. [11].
All calculations are based on DFT within the generalized gradient approximation exchange-
correlational functional as suggested by Perdew, Burke, and Ernzerhof (PBE) [12], using the
projector augmented wave (PAW) technique [13] as implemented within VASP [14, 15]. We
used a plane wave energy cutoff of 400 eV and the Monkhorst-Pack scheme [16] for integration
of the Brillouin zone. For each considered phase the total energy is converged with respect to
k-point sampling to within 0.2 meV/atom, e.g. for MB
2-x
we used a 5×5×5 and 11×11×11 k-
grids for 4×4×3 and 2×2×2 unit cells, respectively. Each phase was relaxed in terms of unit-cell
volume, c/a ratio (when necessary), and internal atomic positions. Structures with disordered
vacancies do break an initially assigned hexagonal crystal symmetry, though after complete
relaxation there is no significant deviation from such complete symmetry. Since magnetism is
beyond the scope of the present work, spin-polarization has been neglected throughout this
study, although, e.g., CrB
2
has been shown to exhibit a helicoidal magnetic structure [17].
However, consideration of magnetism would only influence a potential quantification of
calculated energies, and not the here investigated trends.
The chemical bonding was investigated in terms of projected crystal orbital Hamiltonian
populations (pCOHP) which were derived using the LOBSTER program [18-20]. Using this
method the calculated band-structure energy is reconstructed into orbital interactions. Positive