




Did you find this useful? Give us your feedback










11,576 citations
10,058 citations
6,709 citations
1,944 citations
If both the stem cell283 proliferation rate β and differentiation rate δ are unregulated and hence constant, the authors have284 dS(t)/dt = βS − δS, which for any non-trivial steady-state requires β = δ.
Because the second term of D(t) grows without bounds, the authors may for sufficiently big values of214 t neglect the decaying first term of D(t).
Because model 1 may show oscillations during its relaxation, an analytical calculation of720 its defects is theoretically possible, but leads to expressions too complicated to handle and721 meaningfully interpret.
Because the differentiated cells are responsible for carrying out the designated function499 of a tissue [1], keeping their density as constant as possible is biologically desirable and500 thus grants systems with indirectly regulated stem cell compartments an advantage – even501 more so in tissues like the intestinal and colon epithelium which are constantly exposed to502 mechanical, chemical and biological insults [9].503504
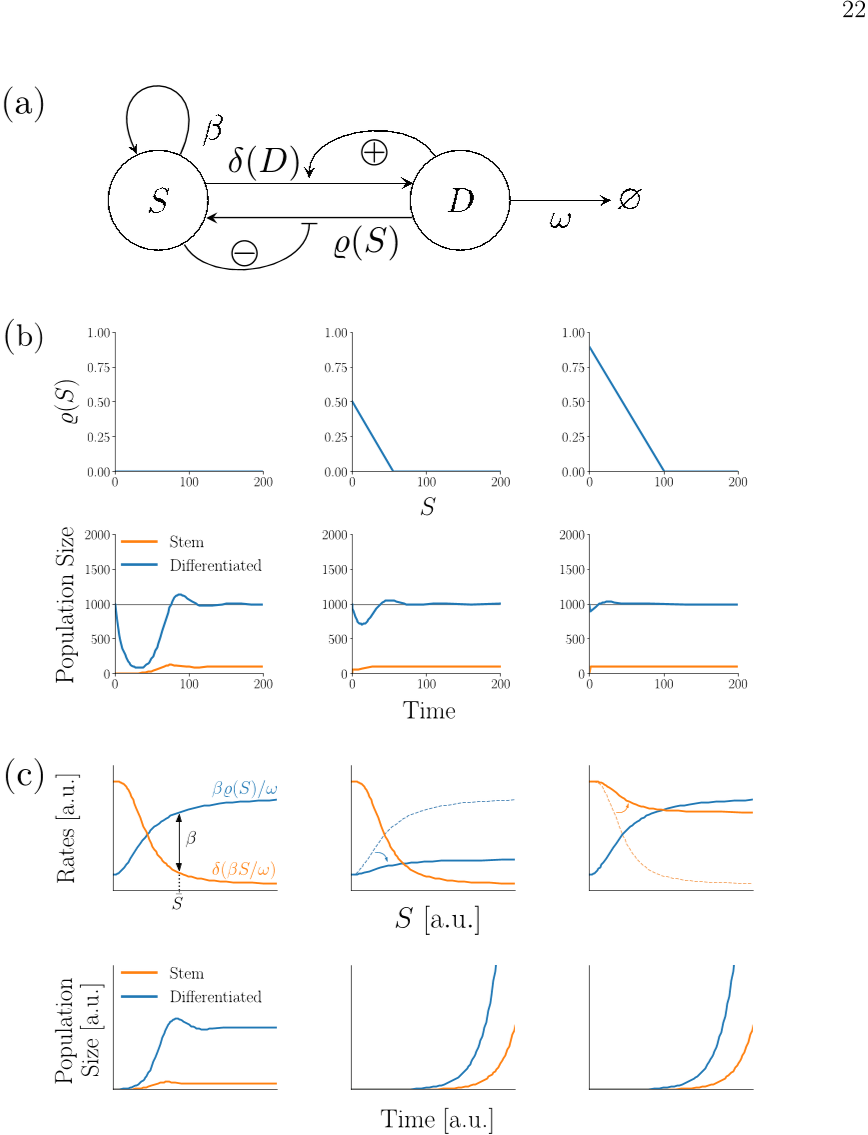
notice that δ0 > 0, δslope > 0 can be made arbitrarily small without ever losing stability and switching to unbounded growth; however, if δ0 exceeds β, the non-trivial steady-state becomes unfeasible and unstable, and the system will always converge to the trivial extinction steady-state (lower two panels). (e) A steeper feedback functions causes stronger and longer oscillations.
For simplicity, the authors chose a piecewise linear function with a positive intercept230 δ0 > 0, denoting the basal differentiation rate of stem cells in the absence of any external231 cues.
Two of them, namely those where the apoptosis rate of differentiated cells is491 controlled, cannot exhibit homoeostasis, showing that a mechanism controlling cycling stem492 cells is required for stability, be it in the form of controlling their proliferation rate, their493 differentiation rate, or potentially both.
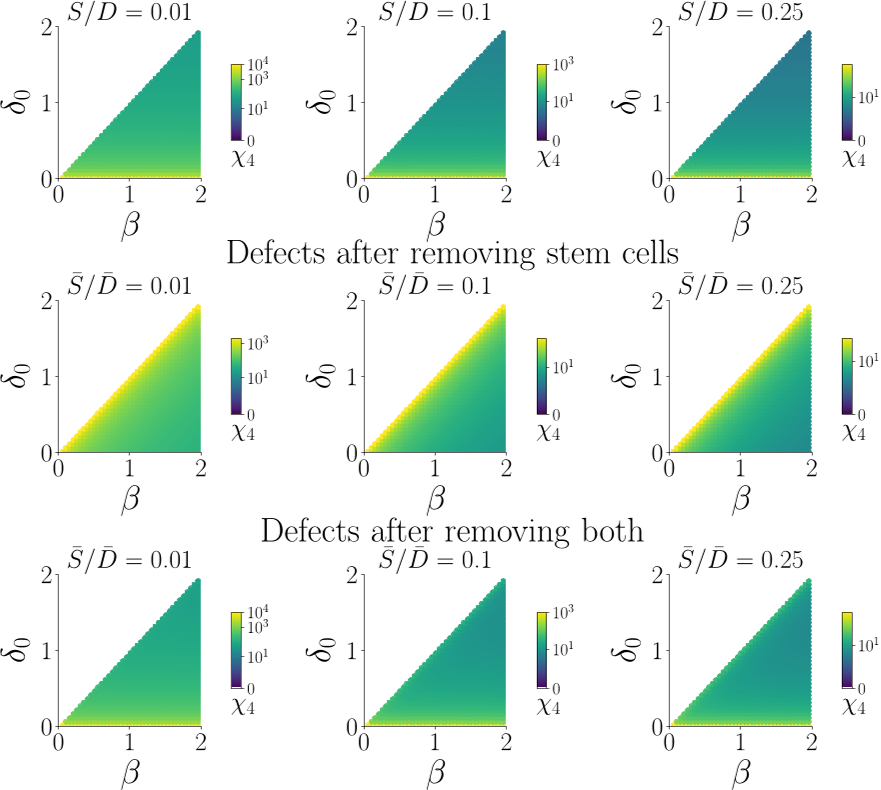
However in case of removing stem cells, the513 colon model very often performed significantly worse since removing stem cells will not514 affect stem cell cycling and stem cell differentiation rates in the colon epithelium model.
The relationship between function slope and oscillatory behaviour of the system is a bio-266 logically interesting result, as it suggests that in order to keep the occurring oscillations in267 check, a smaller slope of the feedback function might be desirable.
An interesting avenue for future research lies in the examination of healthy and tumoural56226intestinal epithelial tissue on the single-cell level in order to answer the question, whether563 the authors are indeed able to identify similar cellular subpopulation and differentiation gradients564 in both of them.
This makes sense because removing509 stem cells from the colon epithelium model cells will cause stem cells to differentiate more510 slowly.
A. Marciniak-Czochra, T. Stiehl, A. D. Ho, W. Jäger, and W. Wagner, Modeling of asymmetric644cell division in hematopoietic stem cells—regulation of self-renewal is essential for efficient645repopulation, Stem cells and development 18, 377 (2009).64629[25]
The authors have shown both analytically and by526 exemplary numerical simulations how this enables the model to recover more gracefully from527 perturbations, especially those reducing the number of stem cells.
By means of a Taylor expansion around the steady-state, the authors can also generalise this finding433 to arbitrary decreasing differentiable functions %.434435 Panel (b) of Figure 4 shows some exemplary numerical simulations of their colon epithe-436 lium model for the case of no dedifferentiation (first column), a linear dedifferentiation437 with %0 = 0.5, %slope = −0.01 (second column) and a faster linear dedifferentiation with438 %0 = 0.9, %slope = −0.01.
Because the two models have different system parameters (β, δ0 vs. β0, δ) we374 cannot directly compare them pointwise in parameter space like the authors did before.