
NOTICE: This is the author’s version of a work that was accepted for
publication in Applied Catalysis B: Environmental. Changes resulting
from the publishing process, such as peer review, editing, corrections,
structural formatting and other quality control mechanisms may not
be reflected in this document. Changes may have been made to this
work since it was submitted for publication. A definitive version was
subsequently published in Applied Catalysis B: Environmental,
Volumes 142–143, October–November 2013, Pages 729–735.
http://doi.org/10.1016/j.apcatb.2013.06.004

Manganese oxides at different oxidation states for heterogeneous activation of
peroxymonosulfate for phenol degradation in aqueous solutions
Edy Saputra
1,2
, Syaifullah Muhammad
1,3
, Hongqi Sun
1
, Ha-Ming Ang
1
, Moses O. Tadé
1
, Shaobin
Wang
1,
*
1
Department of Chemical Engineering and CRC for Contamination Assessment and Remediation of
the Environment (CRC-CARE), Curtin University, GPO Box U1987, Perth, WA 6845, Australia
2
Department of Chemical Engineering, Riau University, Pekanbaru 28293, Indonesia
3
Department of Chemical Engineering, Syiah Kuala University, Banda Aceh, Indonesia
Abstract
A series of manganese oxides (MnO, MnO
2
, Mn
2
O
3
and Mn
3
O
4
) were synthesized and tested in
heterogeneous activation of peroxymonosulfate (PMS) for phenol degradation in aqueous solutions.
Their properties were characterized by several techniques such as X-ray diffraction (XRD),
thermogravimetric analysis (TGA),
scanning electron microscopy (SEM), and N
2
adsorption/desorption isotherms. Catalytic activities of Mn oxides were found to be closely related
to the chemical states of Mn. Mn
2
O
3
is highly effective in heterogeneous activation of PMS to
produce sulfate radicals for phenol degradation compared with other catalysts (MnO, MnO
2
, and
Mn
3
O
4
). The activity shows an order of Mn
2
O
3
> MnO > Mn
3
O
4
> MnO
2
. Mn
2
O
3
could completely
remove phenol in 60 min at the conditions of 25 ppm phenol, 0.4 g/L catalyst, 2 g/L PMS, and 25
o
C. After heat regeneration, the activity could be fully recovered. A pseudo first order model would
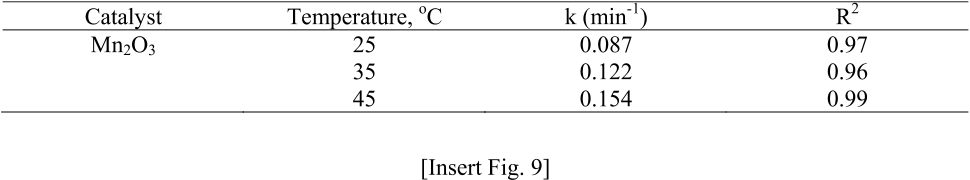
fit to phenol degradation kinetics and activation energy was obtained as 11.4 kJ/mol.
Key words: Mn oxides, peroxymonosulfate activation, advanced oxidation, phenol degradation
*Correspondence author. Email: Shaobin.wang@curtin.edu.au

2
1. Introduction
Over the last decades, water treatment plays an important role in our lives, because of fresh water
crisis and the increasing awareness of human health and ecological systems as a result of industrial
waste pollution. Industrial activities generate large amounts of organic hazardous substances
discharged into the environment. The organic wastes can be found in many industries as by-
products such as petroleum refining, petrochemical, pharmaceutical, plastic, pesticides, chemical
industries, agrochemicals, and pulp and paper industries [1, 2]. The organic pollutants e.g. phenol,
are toxic and cause considerable damage and threat to the ecosystem in water bodies and to the
human health even at low concentrations[3]. It is important to dispose of wastewater in a proper
way in order to comply with environmental regulations. However, the organics in wastewaters from
chemical and related industries cannot be well treated by conventional processes due to degradation
of these pollutants being very slow or ineffective and not environmentally compatible [4, 5]. The
most promising method for degradation of organic pollutants in wastewater is advanced oxidation
processes (AOPs). AOPs are based on generation and utilization of reactive species, such as
hydroxyl radicals (HO•) that have a high standard oxidation potential and react none selectively [6,
7]. Heterogeneous catalytic oxidation systems have recently attracted much interest due to easily
recovery and reuse of the catalysts [8].
Lately, manganese oxides
,
such as MnO, MnO
2
, Mn
2
O
3
and Mn
3
O
4
,
have attracted much attention
due to their physical and chemical properties for being used as catalysts, adsorbents,
supercapacitors, and battery materials [9-15]. Kim and Shim [16] have conducted a study on the
catalytic combustion of aromatic hydrocarbons (benzene and toluene) on manganese oxides. The
results indicated that the catalysts showed high activity in the oxidation of hydrocarbons at
temperatures below 300
o
C. Furthermore, the reactivity of catalysts exhibited an order of Mn
3
O
4
>
Mn
2
O
3
> MnO
2
, which was correlated with oxygen mobility on the catalysts. Ramesh et al. [17]
have studied CO oxidation over a series of manganese oxide catalysts and found that Mn
2
O
3
is the
best catalyst, with the sequence of catalytic activity as MnO ≤ MnO
2
< Mn
2
O
3
. Santos et al. [18]
reported the synthesis of manganese oxide nanoparticles for ethyl acetate oxidation. Complete
oxidation of ethyl acetate was achieved at temperature below 300
o
C. However, few investigations
have been conducted in the activity of a series of manganese oxides at different valence states in
water treatment.
In the most of previous investigations in water treatment, MnO
x
was usually used for Fenton-like
reaction for production of hydroxyl radicals from H
2
O
2
and oxidation of organic compounds.
Recently, sulfate radicals (SRs) produced by Co
2+
/oxone(peroxymonosulfate, PMS) or Ru
3+
/oxone

3
have attracted intense attention in degradation of organic compounds for water treatment [19, 20].
However, Co
2+
or Ru
3+
may generate secondary pollution [21-23]. Therefore, alternative metal such
as Fe
2+
, has been proposed by Zazo et al. [24]. They found that Fe
2+
/H
2
O
2
have a high catalytic
activity for degradation of phenol. In contrary, a recent study by Watts et al. [25] revealed that
Mn
2+
/H
2
O
2
was significantly more reactive than Fe
2+
/H
2
O
2
. Moreover, they found that catalytic
activity was influenced significantly by pH. Saputra et al. [26] reported the oxidative removal of
phenol from water by MnO
2
and studied the factors influencing the reactions. They found that
MnO
2
exhibited as a promising chemical agent under certain conditions for phenol removal from
wastewater. However, no further investigation has been reported for solid MnO
x
for the activation
of PMS to generate SRs.
In this research, we investigate the performance of a series of manganese oxides at varying valence
states for heterogeneous generation of SRs for chemical mineralization of phenol in the solution.
These catalysts will be an alternative for heterogeneous AOP. Several key parameters in the kinetic
study such as phenol concentration, catalyst loading, PMS concentration and temperature were
investigated. Regeneration of used catalysts was also investigated.
2. Experimental methods
2.1. Preparation of Mn catalysts
A manganese dioxide (MnO
2
) was purchased from Sigma-Aldrich Company and used without
further treatment. Mn
2
O
3
was obtained by treating the MnO
2
at 550
o
C in air for 5 h. In addition,
MnO
2
was calcined at 950
o
C in air for 2 h to get Mn
3
O
4
. Another catalyst (MnO) was obtained by a
two-step method. First, MnCO
3
was synthesized by a hydrothermal method [27] and then
calcination was made. Typically, KMnO
4
(3 mmol) and an equal amount of glucose were put into
distilled water at room temperature to form a homogeneous solution, which was transferred into a
45 mL Teflon-lined autoclave. The autoclave was sealed and maintained at 150
o
C for 10 h, and
was then cooled down to room temperature naturally. The resulted solid product (MnCO
3
) was
filtered, washed with distilled water and dried in air at 100
o
C overnight. Finally, MnO catalyst was
obtained by calcination of MnCO
3
at 500
o
C under argon flow at the rate 60 mL/min for 2 h.
2.2. Characterization of catalysts
Catalysts were characterized by X-ray diffraction (XRD), N
2
adsorption/desorption isotherm,
scanning electron microscopy (SEM) and thermogravimetric analysis (TGA). XRD patterns were
obtained on a Bruker D8 (Bruker-AXS, Karlsruhe, Germany) diffractometer using filtered Cu Kα

4
radiation source (λ = 1.54178 Å), with accelerating voltage 40 kV, current 30 mA and scanned at 2θ
from 5 to 70
o
. N
2
adsorption/desorption was measured using a Micromeritics Tristar 3000 to obtain
pore volume and the Brunauer-Emmett-Teller (BET) specific surface area. Prior to measurement
the samples were degased at 120
o
C for 5 h under vacuum condition. The external morphology and
chemical compositions of the samples were observed on a ZEISS NEON 40EsB scanning electron
microscope (SEM) equipped with an energy dispersive spectrometer (SEM-EDS).
2.3. Kinetic study of phenol oxidation
The catalytic oxidation of phenol was carried out in a 1 L glass beaker containing 25-100 ppm of
phenol solutions (500 mL), which was attached to a stand and dipped in a water bath with a
temperature controller. The reaction mixture was stirred constantly at 400 rpm to maintain a
homogenous solution. A fixed amount of peroxymonosulfate (using Oxone, Dupont’s triple salt,
2KHSO
5
•KHSO
4
•K
2
SO
4
(PMS), Sigma-Aldrich) was added into the solution and allowed to
dissolve completely before reaction. Further, a fixed amount of catalyst was added into the reactor
to start the oxidation reaction of phenol. The reaction was carried on for 120 min and at a fixed time
interval, 0.5 mL of solution sample was taken from the mixture using a syringe with a filter of 0.45
µm and then mixed with 0.5 mL methanol to quench the reaction. Concentration of phenol was
analyzed using a HPLC with a UV detector at wavelength of 270 nm. The column used was C-18
with a mobile phase of 30% acetonitrile and 70% ultrapure water. For selected samples, total
organic carbon (TOC) was obtained using a Shimadzu TOC-5000 CE analyzer. For the
measurement of TOC, 5 mL solutions were extracted at a fixed interval and quenched with 5 mL of
3 M sodium nitrite solution and then analyzed on the TOC analyzer.
For recycled catalyst tests, two regeneration methods were used. One is simple washing treatment
and the other is high-temperature calcination. In general, Mn oxides were collected by filtration
after reaction, washing with water and drying at 80 ºC overnight for reuse test. Some dried samples
were further calcined at 500 ºC in air for 1 h and then reused for test again.
3. Result and discussion
3.1. Characterization of manganese oxide catalysts
MnO
2
and MnCO
3
were studied by TGA under air and argon atmosphere, respectively (Fig. 1). The
TGA pattern of MnO
2
(Fig. 1A) shows 5% weight loss below 300
o
C, which corresponds to a loss
of surface adsorbed water, organic and trace amount of oxygen. At around 550
o
C, weight loss of
about 8% corresponds to the loss of oxygen from MnO
2
lattice resulting in the phase transformation


![Fig. 7 Effect of oxone concentration on phenol removal. Reaction condition: [Phenol] = 25 ppm, catalyst (Mn2O3) = 0.4 g/L, and T = 25 oC.](/figures/fig-7-effect-of-oxone-concentration-on-phenol-removal-5e3vz59b.png)
![Fig. 8 Effect of temperature on phenol removal. Reaction condition: [Phenol] = 25 ppm, catalyst (Mn2O3) = 0.4 g/L, and PMS = 2 g/L.](/figures/fig-8-effect-of-temperature-on-phenol-removal-reaction-34tfo4ng.png)


![Fig. 4 Phenol removal efficiency in catalytic oxidation using a series of manganese oxides. Reaction condition: [Phenol] = 25 ppm, catalyst = 0.4 g/L, PMS = 2 g/L, and T = 25 oC.](/figures/fig-4-phenol-removal-efficiency-in-catalytic-oxidation-using-2yf49fon.png)




![Fig. 7 Effect of oxone concentration on phenol removal. Reaction condition: [Phenol] = 25 ppm, catalyst (Mn2O3) = 0.4 g/L, and T = 25 oC.](/figures/fig-7-illustrates-the-effect-of-pms-concentration-on-phenol-t0hdfiue.png)
![Fig. 6 Effect of catalyst loading (Mn2O3) on phenol removal. Reaction condition: [Phenol] = 25 ppm, PMS = 2 g/L, and T = 25 oC.](/figures/fig-6-effect-of-catalyst-loading-mn2o3-on-phenol-removal-228nl2gr.png)
