




Did you find this useful? Give us your feedback












114 citations
39 citations
33 citations
33 citations
32 citations
68 citations
52 citations
31 citations
22 citations
20 citations
because of the very slow rates in heating the samples, the ASC measurements result in the equilibrium temperature dependence of the enthalpy.
By keeping P or Ṫ constant, while increasing or decreasing the temperature of the sample (P and Ṫ positive or negative), four practical modes of operation are obtained.
Measurements have been performed at very slow heating and cooling rates, typically three orders of magnitude slower than the ones usually applied in differential scanning calorimetry (DSC).
In this paper the authors present adiabatic scanning calorimetry (ASC) as an interesting complementary tool to measure simultaneously the temperature dependence of the enthalpy as well as of the heat capacity near the phase transitions in PCMs.
With ASC the problems with superheating or supercooling can in many cases be avoided and true equilibrium data can be obtained by using very slow rates as slow as 2 to 3 orders of magnitude slower than in DSC.
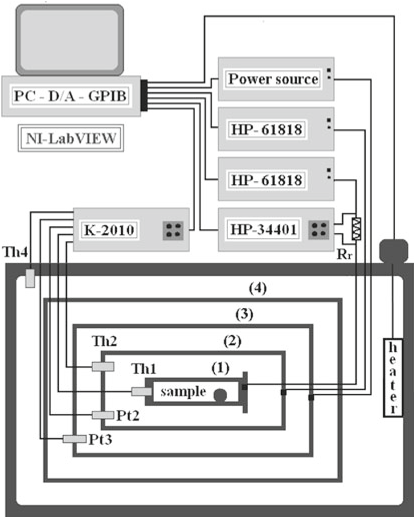
After a long temperature stabilization time of stage 2 (shield around the sample cell) with zero power to the cell (stage 1), the cell attains the same temperature (within a few tenths of a mK).
For PX 42 their transition temperatures (44.5 ◦C for heating and 43.7 ◦C for cooling) are at the upper edge of the 38 ◦C to 43 ◦C range of the manufacturer.
In this paper, ASC is introduced as a suitable tool for simultaneous measurements of the temperature dependence of the enthalpy and of the (effective) heat capacity of PCMs near their solid–liquid phase transition.
Efforts to (partly) overcome these problems for latent heat measurements of PCMs have resulted in running a DSC in an isothermal step mode and/or by applying a T -history method [4].