Q2. How many different parkin mutations have been reported from PD patients?
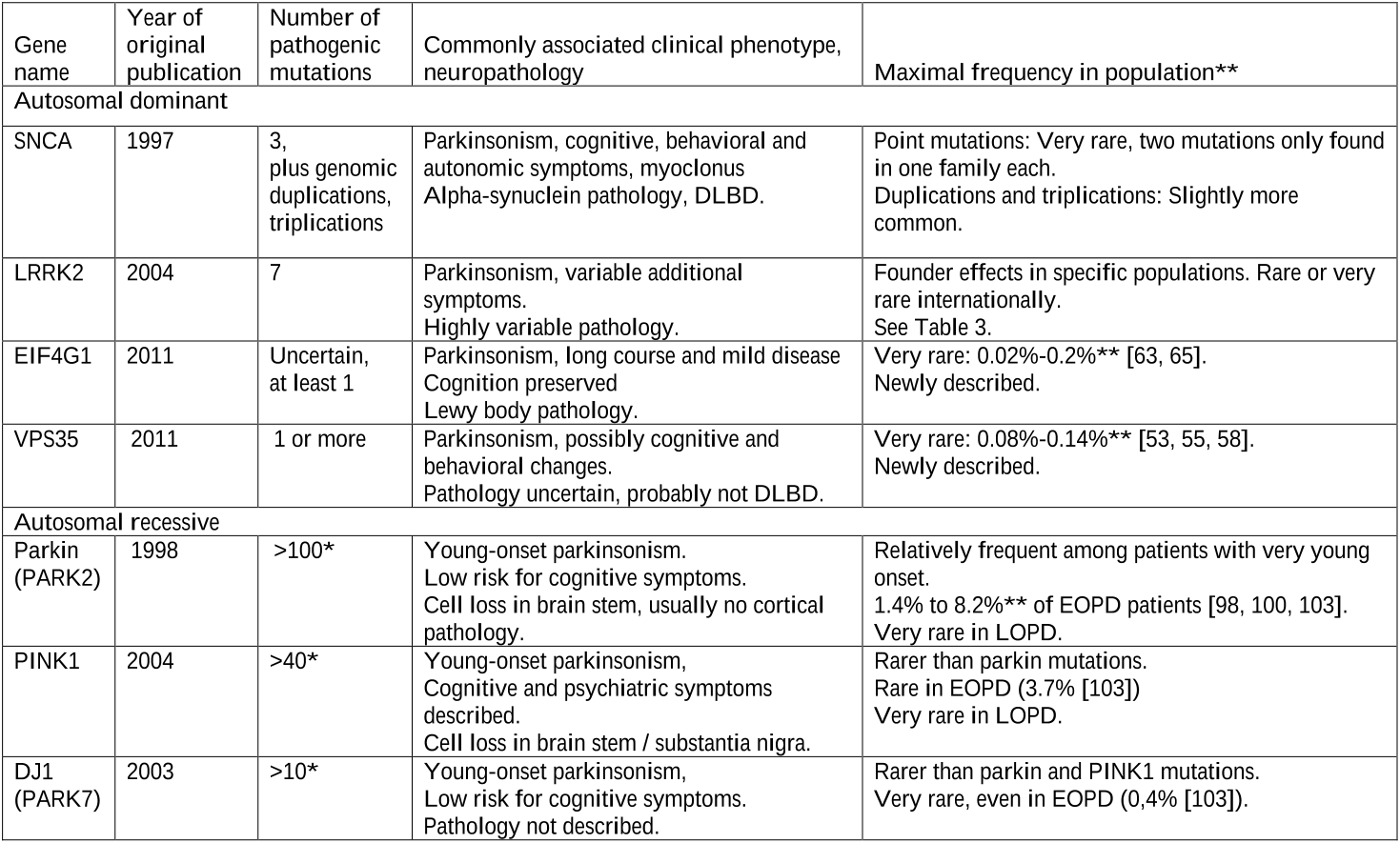
More than 100 different parkin mutations have been reported from PD patients, including copy number variations (deletions, insertions, multiplications), missense and truncating mutations [101].
Q3. What is the common mutation in PD?
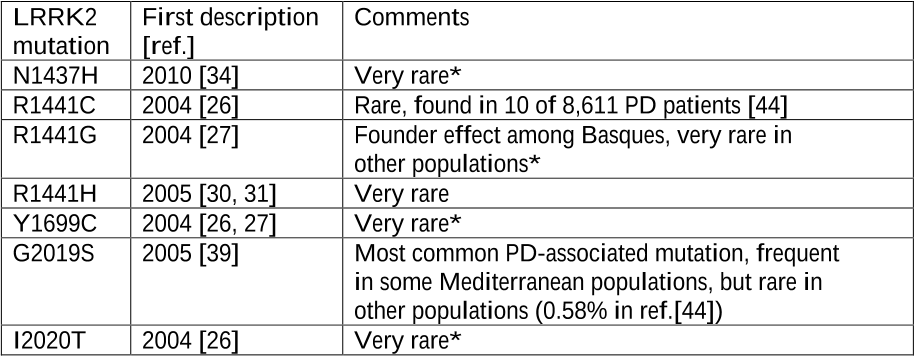
In a recently published international multicenter study, only 49 of 8,371 (0.58%) PD patients of European and Asian origin carried a LRRK2 G2019S mutation [44].
Q4. How many people with PD have parkin mutations?
Other studies found homozygous or compound heterozygous parkin mutations in a lower percentage of patients with early onset-PD (before 40 or 45 years), ranging from 8.2% in Italy, 2.7% in Korea, 2.5% in Poland, to 1.4% in Australia [8, 98-100].
Q5. What are the common causes of parkinsonism?
In rare patients, parkinsonism has been the presenting or predominant clinical manifestation of GRN mutation [77], but mutations in MAPT or GRN are not considered a major cause of familial parkinsonism, especially in the absence of other clinical signs and symptoms.
Q6. How many siblings have a 25% chance of inheriting the same allele?
In families with recessive patterns of inheritance, cosegregation analysis is often limited to a few siblings, but siblings have a 25% chance probability to have inherited the identical allele.
Q7. What is the effect of levodopa on PD?
12Puschmann: Review monogenic PDFeatures common to patients with parkin mutations and PD, aside from young or very young age at onset, are probably a good and lasting effect of levodopa, albeit with the occurrence of dyskinesias during the disease course, and a lower risk for non-motor symptoms such as cognitive decline and dysautonomia [102].
Q8. What are the phenotypes caused by SNCA or the recessive?
The phenotypes caused by mutations in SNCA or the recessive PD genes (parkin, PINK1, DJ1) represent characteristic subtypes of PD.
Q9. How many cases of PD have been identified in Belgium?
Given the prevalence of D620N of 0.14% among PD patients in the two initial studies and of 0.4% in the multicentre study [58], and the fact that replication studies in Belgium [61] or China [62] have not identified additional cases, this mutation is rare.
Q10. What was the earliest reported case of PD in the Austrian family?
Late-onset PD was reported in the Austrian family, one member had only developed depression and tremor with a pathological DATscan indicating incipient PD [55].
Q11. what is the probability of finding a pathogenic mutation in a patient with parkinso?
Genetic testing can today be offered to a subset of patients with unusually young onset, dominant inheritance, and/or a clinical phenotype suggesting a defined monogenic form of parkinsonism.
Q12. What are the common causes of PD?
Carriers of mutations in the other genes may develop parkinsonism with or without additional symptoms, but rarely a disease resembling PD.