1
Multi-route respiratory infection: when a transmission route
may dominate
Caroline X. Gao
1,2.3
, Yuguo Li
4,6
, Jianjian Wei
5,6
*, Sue Cotton
1,3
, Matthew Hamilton
1
,
Lei Wang
5
, and Benjamin J. Cowling
7
1
Centre for Youth Mental Health, University of Melbourne, Parkville, VIC 3052,
Australia
2
School of Public Health and Preventive Medicine, Monash University, 553 St Kilda
Rd, Melbourne, VIC 3004, Australia.
3
Orygen, Parkville, VIC 3052, Australia
4
Department of Mechanical Engineering, The University of Hong Kong, Pokfulam
Road, Hong Kong SAR, China, 999077
5
Institute of Refrigeration and Cryogenics, and Key Laboratory of Refrigeration and
Cryogenic Technology of Zhejiang Province, Zhejiang University, Hangzhou, China,
310000
6
HKU Shenzhen Institute of Research and Innovation, Shenzhen, China, 518053
7
School of Public Health, The University of Hong Kong, Pokfulam Road, Hong Kong
SAR, China, 999077
*Corresponding author:
Jianjian Wei
Institute of Refrigeration and Cryogenics
Zhejiang University
Hangzhou, China, 310000
Tel: (86) 571-87953944, Email: weijzju@zju.edu.cn
Word count : 3728
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted April 11, 2020. ; https://doi.org/10.1101/2020.04.06.20055228doi: medRxiv preprint
NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice.

2
Multi-route respiratory infection: when a transmission route
may dominate
Abstract
The exact transmission route of many respiratory infectious diseases remains a subject
for debate to date. The relative contribution ratio of each transmission route is largely
undetermined, which is affected by environmental conditions, human behavior, the
host and the microorganism. In this study, a detailed mathematical model is developed
to investigate the relative contributions of different transmission routes to a multi-
route transmitted respiratory infection. It is illustrated that all transmission routes can
dominate the total transmission risk under different scenarios. Influential parameters
considered include dose-response rate of different routes, droplet governing size that
determines virus content in droplets, exposure distance, and virus dose transported to
the hand of infector. Our multi-route transmission model provides a comprehensive
but straightforward method to evaluate the transmission efficiency of different
transmission routes of respiratory diseases and provides a basis for predicting the
impact of individual level intervention methods such as increasing close-contact
distance and wearing protective masks. (Word count: 153)
Keywords
Multi-route transmission, short-range airborne route, long-range airborne route,
building ventilation, respiratory infection, influenza
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted April 11, 2020. ; https://doi.org/10.1101/2020.04.06.20055228doi: medRxiv preprint

3
Highlights
1. A multi-route transmission model is developed by considering evaporation and
motion of respiratory droplets with the respiratory jet and consequent exposure of
the susceptible.
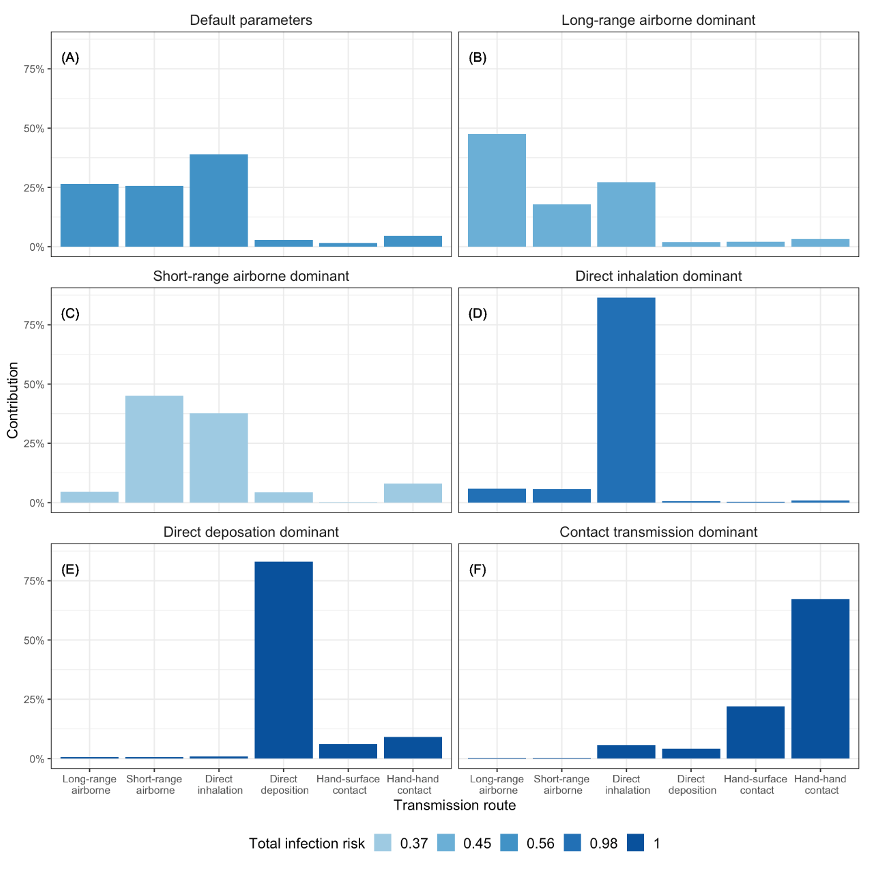
2. We have illustrated that each transmission route may dominate during the
influenza transmission, and the influential factors are revealed.
3. The short-range airborne route and infection caused by direct inhalation of
medium droplets are highlighted.
Introduction
The 2003 Severe Acute Respiratory Syndrome (SARS) epidemics, the 2009 H1N1
influenza (Swine Flu) pandemic, the 2015 Middle East Respiratory Syndrome –
coronavirus (MERS-CoV) epidemics and the ongoing novel human coronavirus
(SARS-CoV-2) global pandemic have all highlighted the importance of studying the
transmission mechanism of respiratory infectious diseases (1-4).
Respiratory diseases are often simply assumed to be transmitted via “close contact”;
however, the transmission mechanisms are complex involving more than one
transmission route including direct or indirect contact, large droplet, and airborne
routes (5-9). There are many physical (respiratory particles and droplets generation),
virological (viral loading, survival, location of virus receptor, etc.), behavioral
(exposure distance, frequency of handshaking and surface touching, etc.) and
environmental factors (temperature, humidity, ventilation, etc.) that affect the
transmission (8, 10).
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted April 11, 2020. ; https://doi.org/10.1101/2020.04.06.20055228doi: medRxiv preprint

4
Hence, respiratory infections may show various characteristics under different contact
scenarios. For example, airborne transmission was identified as played the leading
role in an influenza outbreak on a commercial aircraft in 1977 in Alaska (11).
Conversely, in a H1N1 outbreak in a tour group in China, close contact was the most
correlated factor with the transmission (12). Conflicting evidence for transmission
routes, like these two cases, are prevalent for almost all respiratory infectious diseases
(8). Failure in understanding the complex multi-route transmission mechanisms leads
to recommendations of more conservative intervention methods such as keep a
distance rather than increasing ventilation and wearing masks. The consequences of
more conservative interventions can be catastrophic such as the global pandemic of
SARS-CoV-2 outbreak (13-15).
However, understanding of multi-route transmission is by no means an easy task.
Findings from animal challenge models are difficult to extrapolate to human
transmission (16). Human challenge models are expensive and often unethical (17).
Observational studies of existing outbreaks often fail to capture important time
relevant evidence. A more feasible approach is to use mathematical models to
describe the multi-route transmission using known parameters such as droplet
generation rate, virus shedding rate, and virus survival rates. A few mathematical
studies have developed multi-route transmission models such as by Nicas and Jones
(18), Atkinson and Wein (19) and Spicknall and colleagues (20). However, many
critical factors, such as evaporation of respiratory droplets, travelling of the large
droplets in the respiratory jet, pulmonary deposition, dose-response rate for different
route were not fully evaluated in these models, which may underestimate the role of
smaller droplets.
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted April 11, 2020. ; https://doi.org/10.1101/2020.04.06.20055228doi: medRxiv preprint

5
In this paper, we first provide comprehensive definitions of transmission routes which
incorporates the underlying physical principles of multi-route transmission. Second,
we establish a more advanced simulation model to describe the infection via different
routes considering the physical components of particles and droplets movement,
differences in possible viral dose-effect as well as human-behavior factors such as
touching mouth and nose. We use influenza as an example due to the large number of
related studies for extracting modelling parameters. Using the model, we aim to
challenge the traditional dichotomous thinking of close contact transmission vs
airborne (aerosol) transmission via highlighting the scenarios under which each
transmission route may dominate and how environmental and behavior factors
interact in the transmission mechanism.
Methodology
Transmission routes definitions
Traditional definitions for transmission routes include the airborne route (also referred
as aerosol transmission) (21), large droplet route, and contact route (6, 22). However,
such definitions are somewhat ambiguous. Firstly, the cut-off size of droplets for
airborne transmission has always been controversial (8, 22, 23). The droplet nuclei,
first defined by Wells (24), refers to the residues of droplets after complete
evaporation. Centers for Disease Control and Prevention (CDC) defined the cut-off
size of 5 µm for airborne transmission (25), and the threshold distance for airborne
transmission is defined by the World Health Organization (WHO) as 1.0 m (26).
However, it is known that droplet nuclei over 5 µm may also easily suspend and
disperse over 1.0 m to cause transmission of respiratory disease, depending on the
All rights reserved. No reuse allowed without permission.
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
The copyright holder for this preprintthis version posted April 11, 2020. ; https://doi.org/10.1101/2020.04.06.20055228doi: medRxiv preprint