!
1!
The origin of bistability in the butyl-substituted spiro-
biphenalenyl-based neutral radical material
!
Maria Fumanal
¶
, Juan J. Novoa, Jordi Ribas-Arino*
Departament de Química Física and IQTCUB, Facultat de Química, Universitat
de Barcelona, Av. Diagonal 645, 08028-Barcelona (Spain)
* jordi.ribas.jr@gmail.com, j.ribas@ub.edu
¶
Present address: Laboratoire de Chimie Quantique, Institut de Chimie UMR7177
CNRS-Université de Strasbourg, 1 Rue Blaise Pascal BP 296/R8, F-67007 Strasbourg,
France
!
!

!
2!
!
Abstract
One of the most remarkable bistable materials so far reported is made of π-dimers of a
butyl-substituted spiro-biphenalenyl boron radical (butyl-SBP). The phase transition of
this material, which is accompanied by changes in its optical, conductive and magnetic
properties, occurs with a hysteretic loop 25-K wide and is centered at 335 K. Here, we
present a computational study aimed at deciphering the origin of this hysteresis. We
show that the phase transition of butyl-SBP consists of a spin transition of their
constituent π-dimers coupled with an order-disorder transition involving the butyl
chains linked to the N atoms of the superimposed phenalenyl rings of the π-dimer.
Below 335 K, the terminal methyl group of the butyl chains adopts a gauche
conformation with respect to the methylene unit bonded to the N atom. Above 335 K,
the methyl group is in an anti conformation and exhibits dynamic disorder. The gauche
! anti conformational rearrangement triggers the spin transition of the π-dimers and is
responsible for the hysteretic behavior of butyl-SBP. Specifically, the onset of the
phase transition in the heating mode and, thus, the width of the hysteresis loop, are
governed by the high energy cost and the strong structural cooperative effects
associated with this conformational change. Our results show that coupling a spin
switch with a conformational switch in a molecular crystal provides a promising strategy
in the design of new bistable materials.
!

!
3!
!
Introduction
Bistability is an intriguing phenomenon exhibited by a few materials that present two
stable phases that can both exist within a given range of temperatures. Molecule-
based bistable materials have been the subject of intense research during the last
years because they hold great promise for application in sensors, displays and
switching devices.
1,2,3 ,4 ,5
The numerous examples of molecular bistable materials
include: materials based on transition metal complexes undergoing spin
transitions
6,7,8,9,10,11,12,13,14
, organic spin-transition materials
15,16,17,18,19,20,21,22
, compounds
whose phase transition is induced by a charge transfer between an electron-donor and
an electron-acceptor
23, 24,25 ,26 ,27
, compounds featuring charge-transfer-induced spin
transitions
28 , 29 , 30
, inorganic-organic hybrid frameworks undergoing phase
transitions
31,32
, molecular crystals whose phase transitions are triggered by changes in
the orientation of molecules
33
. The transition temperature and the hysteresis loop width
of a bistable material are crucial parameters in determining whether its bistability can
be harnessed in technological applications. These two parameters, in turn, depend on
the intermolecular interactions within the crystal and on the energy barriers associated
with the lattice reorganization upon phase transition. For most of the bistable
compounds reported so far, very little is known about either the origin of the energy
barriers associated with their phase transitions (i.e, whether the energy barrier of the
overall phase transition is dominated by a single molecular rearrangement or whether
the barrier is the result of the contributions of different reorganization events) nor the
role of structural cooperativity in promoting such phase transitions. It is clear that the
lack of this sort of information poses a major obstacle for the rational design of new
derivatives of a given bistable parent compound with the goal of fine tuning its
transition temperature and its hysteresis loop width. Therefore, the studies aimed at
elucidating the origin of these barriers and at establishing the role of cooperative
effects have the potential to offer most valuable hints on how to devise new bistable
materials with improved properties. Here, on the basis of a computational study, we
disclose the origin of the hysteretic phase transition of a phenalenyl-based butyl-
substituted neutral radical, which is one of the most prominent compounds within the
family of bistable materials.
Phenalenyl (PLY) is an odd-alternant hydrocarbon neutral radical arising from a
triangular fusion of three benzene rings. This open-shell molecule has emerged in the
past years as one of the most versatile building blocks for functional molecular devices

!
4!
and materials.
16,34,35,36,37,38,39,40
The numerous spiro-biphenalenyl (SBP) boron radicals
reported by Haddon and coworkers constitute a very important class of PLY
derivatives.
41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57
SBPs present two nearly perpendicular
phenalenyl units connected through a boron spiro-linkage. The N- and O-functionalized
SBPs (ie, SBPs in which each phenalenyl unit is bonded to the central boron atom via
an oxygen and a nitrogen atom) exhibit diverse packing motifs in the solid state, and
hence different physical properties, depending on the substituents attached to the
nitrogen atom. Ethyl (1) and butyl-substituted (2) SBPs (see Figure 1) present a crystal
structure containing π -dimers as the basic building block (see Figure 2 and Figure S1).
These two compounds undergo a phase transition that is accompanied by a change in
their optical, conductive and magnetic properties.
16,42
The phase transition of ethyl-SBP
is reversible and occurs at about 140 K, while that of butyl-SBP occurs with an
hysteretic loop 25-K wide and is centered at a much higher temperature (~ 335 K). At
this point, it is worth mentioning that butyl-SBP is one of the few multifunctional bistable
materials that switch the response in multiple physical channels upon phase
transition.
25,30,32,26
Besides, the volume of the crystals of butyl-SBP significantly change
upon phase transition; specifically, a notable expansion of the crystal is observed when
the system switches from its low-temperature (LT) phase to its high-temperature (HT)
phase.
58
This volume change in response to external stimuli is currently a sought-after
phenomenon in the context of new functional materials due to its potential applicability
to microscale or nanoscale actuators.
33
The experimental
58,59
and theoretical studies
60,61,62,63,64
conducted over the last years on
ethyl- and butyl-SBP have culminated in a clear understanding of their electronic
structure and the different magnetic and conducting properties of their phases. Upon
phase transition in the heating mode, the constituent π-dimers of these materials
undergo a spin transition from a closed-shell diamagnetic singlet state to an open-shell
paramagnetic state. Below the spin transition temperature, the structures of the π-
dimers are governed by the potential energy surface (PES) of the ground singlet state
(
1
A
g
state), whose minimum structure features a partial localization of the unpaired
electrons of each SBP radical in the superimposed phenalenyl (sup-PLY) rings, that is,
on the phenalenyl (PLY) units directly involved in the π-dimer (see Figure 2a). The
strong coupling between the SBP unpaired electrons in this configuration leads to a
magnetically silent state, and, thus, to a diamagnetic LT phase. Above the spin
transition temperature, the π-dimers adopt a configuration characterized by a
localization of the SBP unpaired electrons in the nonsuperimposed phenalenyl (non-
PLY) units, that is, on the PLYs not directly involved in the π-dimer (see Figure 2b),

!
5!
which leads to a paramagnetic phase. This configuration is exclusively governed by the
PES of the ground triplet state (
3
A
u
state) because the corresponding open-shell singlet
does not feature any minimum in that region of the PES even if it lies slightly below in
energy than the triplet state. In a recent article
64
, we have shown that the high-spin
(HS) state is energetically competitive with the low-spin (LS) state because the
electrostatic component of the interaction energy between SBP radicals in the π-
dimers is more attractive in the high-temperature
3
A
u
state than in the low-temperature
1
A
g
state. This electrostatic stabilization of the high-temperature
3
A
u
state was ascribed
to the zwitterionic nature of the SBP moieties, in particular, to the interaction between
the positively-charged superimposed PLYs in the triplet state (Figure 2b) and the
negatively-charged spiro-linkages with the central boron atom. These electrostatic
interactions also explain why the unpaired electrons prefer to localize on the
nonsuperimposed PLYs in the high-temperature triplet state.
64
Despite the current good understanding of the electronic structure of the π-dimers of
ethyl- and butyl-SBP and several theoretical studies on other phenalenyl-based
systems
65,66,67,68,69,70,71,72,73,74,75,76,77,78
, there are two crucial questions concerning the
phase transitions of ethyl- and butyl-SBP that remain unsettled, namely: i) why is the
transition temperature of butyl-SBP so much higher than that of ethyl-SBP?, and ii) why
does butyl-SBP display an hysteretic phase transition, in contrast with ethyl-SBP,
which features a smooth phase transition? A meticulous study carried out by Haddon
and coworkers in Ref. 58 on numerous crystal structures of butyl-SBP at different
temperatures led to the suggestion that the HT phase is the thermodynamically stable
phase within the bistability region, while the existence of the LT phase within the
hysteretic loop was rationalized on the basis of the large energy barrier that the system
needs to overcome when switching from LT to HT. Even if this barrier was estimated to
be larger than 24 kcal/mol, the specific molecular rearrangements responsible for that
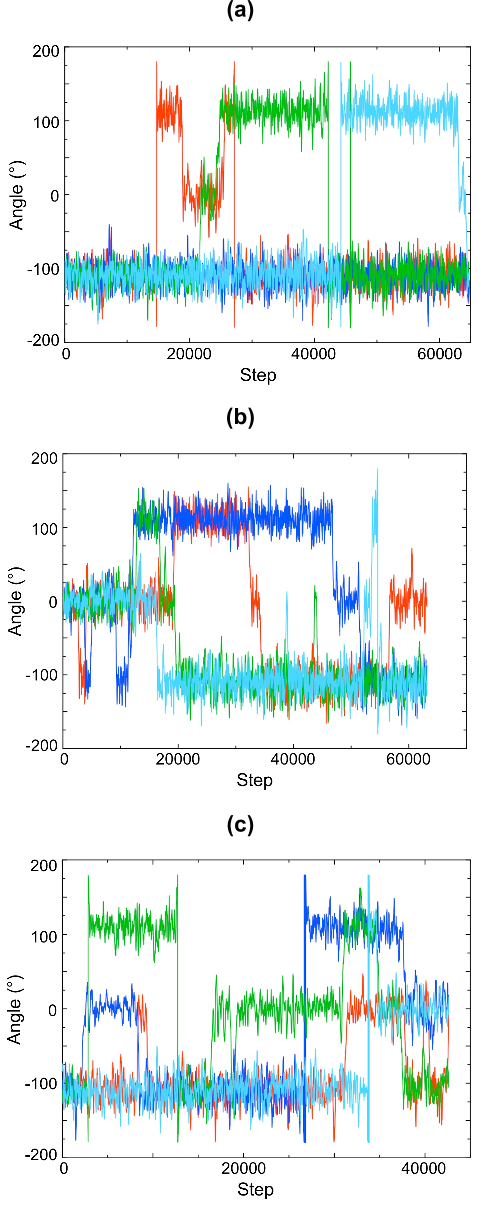
barrier were not identified. In the computational study herein presented, not only do we
provide a rationale for the higher spin-transition temperature of butyl-SBP but also
disclose the hitherto elusive origin of its hysteresis loop. In particular, our study reveals
that the bistability arises from a very simple molecular rearrangement, namely, a
conformational rearrangement of the butyl groups attached to the SBP radicals.