Q2. What is the reaction sequence of the catalyst?
The formation of benzene, as a result of dealkylation reaction predominates on acid ðAl3þÞ sites, whereas on redox sites ðCr5þ=6þÞ oxidation of toluene is taking place leading to benzaldehyde.
Q3. What is the potential of chromium containing solid catalysts?
The incorporation of transition metal ions into the framework sites of microporous aluminosilicates and aluminophosphates has been reported in the literature and resulting systems are potential catalysts for various selective redox reactions [1– 3].
Q4. What is the function of the organic ammonium cation from TMAOH?
The function of organic ammonium cation from TMAOH is probably to modify the strength of the electrostatic interactions between the aluminophosphate species and the cationic surfactant micelle assembly to form the SþI =TMAþ ion pair.
Q5. What is the potential of chromium containing solid catalysts in the industrial phase?
But for the large-scale production of fine chemicals, replacement of conventional homogeneous systems by heterogeneous catalysts will be advantageous inthe sense of catalyst recovery and reduction of undesirable side reactions.
Q6. What is the potential of chromium containing solid catalysts in liquid phase?
With the recent discovery of mesoporous materials, the activities of chromium containing MCM-41, MCM-48, HMS have been tested for the oxidation of alkylbenzenes with peroxides as oxidants [8–10].
Q7. What is the effect of NaOH on the cationic surfactant?
If either NaOH or NH4OH is used, the smaller cations Naþ, NHþ4 compete with the aluminophosphate species and thus restrict the interaction with the positively charged cationic surfactant.
Q8. What is the reason for the decrease in surface area of the Cr–AlPO compared?
The decrease in the surface area of the Cr– AlPO compared to AlPO could be due to partial loss of crystallinity, which is in agreement with the observation from XRD.
Q9. What is the PII of the chromium catalyst?
PII: S1566-7367 (01 )00070-Xcatalysts can be increased by using molecular oxygen as oxidant and thereby carrying the reaction in vapour phase it is possible to control the leaching of chromium from the framework.
Q10. What is the g value of the calcined Cr–AlPO?
ESR spectra of calcined Cr–AlPO shows g value at 1.95 characteristic of pentavalent chromium in tetrahedral or distorted tetrahedral co-ordination.
Q11. What was the pH of the gel?
The pH of the gel was maintained at 9.5 with tetra methyl ammonium hydroxide, as the use of other sources like NaOH and NH4OH resulted only in the amorphous materials.
Q12. What is the effect of calcination on the aluminophosphate?
As stated earlier, aluminium hydroxide may form a less polymerised aluminophosphate with many hydroxyl groups and favour the assembly of the mesostructure compared to other aluminium sources [11,12].
Q13. What is the potential of chromium containing porous solid catalysts in liquid phase?
the potential of chromium containing porous solid catalysts in liquid phase is limited because of the leaching of the active metal from framework.
Q14. What is the morphology of the AlPO materials?
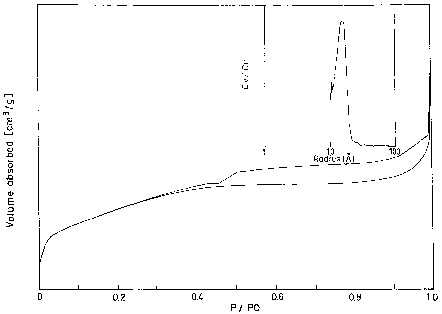
Pore size ( A) Pore volume (cc/g)AlPO 34.5 33.0 38.10 685 28 0.65 Cr–AlPO 35.0 33.4 38.56 490 29 0.51Cr–AlPO materials have been prepared by hydrothermal synthesis and found to have hexagonal MCM-41 like morphology.
Q15. What is the composition of the gel?
Mesoporous chromiumaluminophosphateswere prepared using cetyltrimethylammoniumbromide (CTAB) as surfactant and with the following gel composition.
Q16. What was the diffraction pattern of the sample?
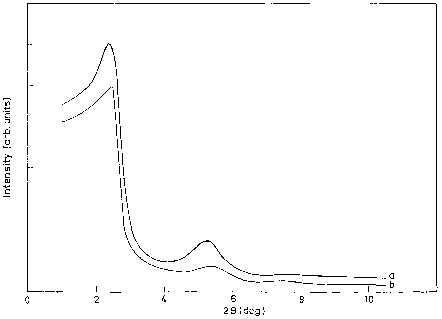
The low angle X-ray diffraction pattern of the sample was recorded on a Siemens D 500 ðh=2hÞ using monochoromatised Cu-Ka radiation ðk ¼ 1:5406 AÞ with a scan speed of 1 /min over the range 2 < 2h < 10 .
Q17. What is the pore size of the AlPO?
In both the cases the pore size is around 29 A.UV–VIS spectroscopy is a technique for the characterisation of transition–metal-incorporated zeolites [13–15].
Q18. What is the g value of the as-synthesised Cr–AlPO?
4. As-synthesised material shows a broad singlet with a g value of 1.98 indicating Cr3þ ions in octahedral co-ordination [20,21].
Q19. What is the chemical composition of the chromiumaluminophosphate?
In this communication, the synthesis, characterisation and catalytic activity of mesoporous Cr–AlPO for toluene oxidation with molecular oxygen are reported.
Q20. What is the XRD pattern of the AlPO?
CO, CO2 and H2O were analysed in a Carboxen-1010 capillary column and analysed by a TCD.XRD patterns of as-synthesised and calcined AlPO, Cr–AlPO are shown in Fig. 1. XRD patterns of AlPO show low angle peaks typical for hexagonal phase.