PDF hosted at the Radboud Repository of the Radboud University
Nijmegen
The following full text is a publisher's version.
For additional information about this publication click this link.
http://hdl.handle.net/2066/118930
Please be advised that this information was generated on 2022-05-30 and may be subject to
change.

Passive targeting of lipid-based nanoparticles
to mouse cardiac ischemia–reperfusion injury
Tessa Geelen, Leonie E. Paulis, Bram F. Coolen, Klaas Nicolay and
Gustav J. Strijkers*
Reperfusion therapy is commonly applied after a myocardial infarction. Reperfusion, however, causes secondary
damage. An emerging approach for treatment of ischemia–reperfusion (IR) injury involves the delivery of therapeutic
nanoparticles to the myocardium to promote cell survival and constructively influence scar formation and myocardial
remodeling. The aim of this study was to provide detailed understanding of the in vivo accumulation and distribution
kinetics of lipid-based nanoparticles (micelles and liposomes) in a mouse model of acute and chronic IR injury. Both
micelles and liposomes contained paramagnetic and fluorescent lipids and could therefore be visualized with magnetic
resonance imaging (MRI) and confocal laser scanning microscopy (CLSM). In acute IR injury both types of nanoparticles
accumulated massively and specifically in the infarcted myocardium as revealed by MRI and CLSM. Micelles displayed
faster accumulation kinetics, probably owing to their smaller size. Liposomes occasionally co-localized with vessels
and inflammatory cells. In chronic IR injury only minor accumulation of micelles was observed with MRI. Nevertheless,
CLSM revealed specific accumulation of both micelles and liposomes in the infarct area 3 h after administration. Owing
to their specific accumulation in the infarcted myocardium, lipid-based micelles and liposomes are promising vehicles
for (visualization of) drug delivery in myocardial infarction. Copyright © 2012 John Wiley & Sons, Ltd.
Supporting information may be found in the online version of this paper
Keywords: liposomes; micelles; myocardial infarction; drug delivery; MRI
1. INTRODUCTION
The best treatment option for acute myocardial infarction is
currently early reperfusion of the affected myocardium. The aim
of restoring blood flow is to limit the extent of myocardial necrosis
and scarring, which are important factors in the development of
systolic heart failure and determine prognosis. The downside
of reperfusion treatment is that it causes adverse secondary
ischemia–reperfusion (IR) injury by the generation of cytotoxic
reactive oxygen species, resulting in additional apoptosis (1). After
the initial IR event a dynamic cascade of events is initiated to
promote myocardial infarct healing. In the acute phase, early after
the ischemic event, infarct healing is characterized by cell death
and inflammation. At later time points, in the chronic phase, the
affected myocardium remodels into scar tissue (2–5).
An emerging approach for further treatment of IR injury
involves the delivery of therapeutic compounds to the myocar-
dium to promote cell survival and constructively influence scar
formation and myocardial remodeling (6,7). The effectiveness
of intravenously injected therapeutics may be hampered by fast
clearance from the blood circulation and by lack of retention in
the infarcted myocardium. To improve drug delivery to the
infarct, nanoparticles offer a suitable vehicle as they can be
designed to display long blood circulation half-lives and are able
to encapsulate a large amount of drug molecules. Furthermore,
owing to their size, nanoparticles demonstrate prolonged
retention in the infarct area, which is enabled by the presence
of leaky vasculature (8,9). Previously, liposomal nanoparticles
were exploited for such purposes. Injection of ATP-, coenzyme
Q10-, PGE
1
- or adenosine-loaded liposomes resulted in a
reduction of the extent of irreversibly damaged myocardium
within the area at risk (10–15). Furthermore, accumulation of
pegylated micelles in the infarcted myocardium has been
demonstrated in a rabbit model of myocardial infarction (16).
Therefore, micelles were proposed for delivery of lipophilic drugs
(16,17). The above studies have in common that information on
accumulation kinetics in the heart was lacking and the exact
location of the lipid-based nanoparticles at cellular scale was
not visualized. Obviously, these need to be determined for
optimization of drug delivery. Furthermore, only acute IR injury
(up to 3 h reperfusion time) was considered, which is clinically
less relevant, and therefore later time points after IR injury
should be studied as well.
Therefore, the aim of this study was to provide detailed under-
standing of the in vivo accumulation and distribution kinetics of
long-circulating lipid-based nanoparticles in a mouse model of
acute (1 day old) as well as chronic (up to 2 weeks) myocardial
IR injury. We studied micelles (diameter approximately 15 nm)
and liposomes (diameter approximately 100 nm) to investigate
influence of nanoparticle size. Both micelles and liposomes were
equipped with paramagnetic Gd-containing lipids and with
fluorescent lipids. Importantly, this enabled us to study the
distribution of the nanoparticles in the infarcted myocardium
in vivo using MRI, as well as to localize the nanoparticles at the
* Correspondence to: G. Strijkers, Biomedical NMR, Department of Biomedical
Engineering, Eindhoven University of Technology, PO Box 513, 5600 MB,
Eindhoven, The Netherlands. E-mail: g.j.strijkers@tue.nl
T. Geelen, L.E. Paulis, B.F. Coolen, K. Nicolay, G.J. Strijkers
Biomedical NMR, Department of Biomedical Engineering, Eindhoven University
of Technology, PO Box 513, 5600 MB, Eindhoven, The Netherlands
Full Paper
Received: 17 May 2012, Revised: 12 July 2012, Accepted: 21 August 2012, Published online in Wiley Online Library:
(wileyonlinelibrary.com) DOI: 10.1002/cmmi.1501
Contrast Media Mol. Imaging 2013, 8 117–126 Copyright © 2012 John Wiley & Sons, Ltd.
117

cellular level ex vivo with confocal laser scanning microscopy
(CLSM). As a control late gadolinium enhancement (LGE) MRI
was performed after administration of Gd–DTPA. Furthermore,
contrast-enhanced in vivo MRI was complemented with cine
MRI to determine cardiac function.
2. RESULTS AND DISCUSSION
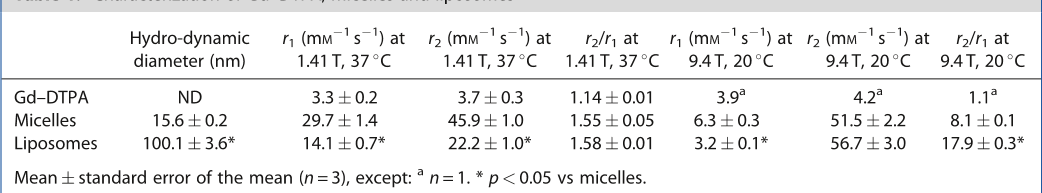
2.1. Nanoparticle Characterization
Micelles and liposomes were first characterized with respect to
hydrodynamic size and MRI relaxivity properties at 1.41 and
9.4 T (Table 1). Gd–DTPA was included as a reference. Micelle
diameter was approximately 15 nm and the liposome diameter
of 100 nm was significantly larger. At 1.41 T, the micelle and
liposome longitudinal relaxivity (r
1
) values were higher than
those for Gd–DTPA. Thus, the nanoparticles are powerful MRI
contrast agents, which is further amplified by the high payload
of Gd–DOTA-carrying lipids incorporated in the lipid membranes.
As expected, at 9.4 T, r
1
values were considerably lower compared
with 1.41 T (18). Nevertheless, the r
1
of micelles was still higher
than the r
1
of Gd–DTPA. The ratio of transversal and longitudinal
relaxivities (r
2
/r
1
) of both micelles and liposomes was relatively
high at 9.4 T, indicating that the nanoparticles will display a
pronounced T
2
shortening effect at high field strengths. From
the relaxivity data we concluded that the nanoparticles possessed
ample sensitivity for in vivo MR imaging of nanoparticle accumula-
tion in cardiac IR injury.
2.2. Blood Circulation Half-lives and Biodistribution
To investigate the blood circulation half-lives and biodistribution,
mice were injected with Gd–DTPA, micelles or liposomes and
blood samples were obtained up to 48 h after administration.
Blood ΔR
1
values (= 1/T
1,post
1/T
1,pre
) determined at 9.4 T served
as a measure of the concentration of nanoparticles in the circula-
tion (Fig. 1a) (19). From mono-exponential fits the blood circulation
half-lives were calculated (Fig. 1a). As expected, Gd–DTPA had a
short blood circulation half-life of 0.30 0.05 h owing to fast renal
clearance enabled by its small size. Micelles and liposomes
displayed relatively long blood circulation half-lives of 3.90 0.44
and 2.31 0.40 h, respectively. Previously, van Bochove et al.
observed longer blood circulation half-lives in mice for micelles
(22.5 2.8 h) and liposomes (7.0 1.0 h) containing neutral
Gd–DTPA-carrying lipids (20). The Gd–DOTA-conjugated lipids
used in this study have a charge of 1, which could induce faster
blood clearance via ingestion by phagocytic cells (21).
To determine the in vivo fate of micelles and liposomes, animals
were killed 48 h after administration of the nanoparticles. Organs
were excised and the biodistribution was studied with CLSM
(Fig. 1b). Micelles accumulated mainly in the kidney, suggesting
a clearance pathway that partly involved renal elimination.
Liposomes were mainly detected in the spleen and in smaller
amounts in the liver, lungs and kidneys. The large size of liposomes
prohibits renal clearance and therefore it is likely that they are
removed from the blood by the reticuloendothelial system.
2.3. In Vivo MRI
Mice underwent transient occlusion (30 min) of the left anterior
descending (LAD) coronary artery to induce cardiac IR injury.
Contrast-enhanced in vivo MRI was performed at day 1 (acute), or
at week 1 or week 2 (chronic) after IR injury to visualize the
distribution and extravasation kinetics of Gd–DTPA, micelles and
liposomes. The MRI protocol is depicted schematically in Fig. 2.
Table 1. Characterization of Gd–DTPA, micelles and liposomes
Hydro-dynamic
diameter (nm)
r
1
(mM
1
s
1
)at
1.41 T, 37
C
r
2
(mM
1
s
1
)at
1.41 T, 37
C
r
2
/r
1
at
1.41 T, 37
C
r
1
(mM
1
s
1
)at
9.4 T, 20
C
r
2
(mM
1
s
1
)at
9.4 T, 20
C
r
2
/r
1
at
9.4 T, 20
C
Gd–DTPA ND 3.3 0.2 3.7 0.3 1.14 0.01 3.9
a
4.2
a
1.1
a
Micelles 15.6 0.2 29.7 1.4 45.9 1.0 1.55 0.05 6.3 0.3 51.5 2.2 8.1 0.1
Liposomes 100.1 3.6* 14.1 0.7* 22.2 1.0* 1.58 0.01 3.2 0.1* 56.7 3.0 17.9 0.3*
Mean standard error of the mean (n = 3), except:
a
n =1. *p < 0.05 vs micelles.
Figure 1. Blood circulation half-lives and biodistribution. (a) ΔR
1
(=1/T
1,post
1/T
1,pre
) of all blood samples, as measured at 9.4 T, plotted vs time after
injection. Blood circulation half-lives were determined by fitting with a mono-exponential decay function (solid lines), leading to the blood circulation
half-lives (t
1/2
) as shown in the table (Mean SD). (b) Biodistribution of nanoparticles in several organs. The red color originates from the near-infrared
(NIR) signal of micelles and liposomes. As a non-fluorescent negative control, organs from mice injected with Gd–DTPA are shown. Scale bar = 100 mm.
T. GEELEN ET AL.
wileyonlinelibrary.com/journal/cmmi Copyright © 2012 John Wiley & Sons, Ltd. Contrast Media Mol. Imaging 2013, 8 117–126
118

In addition, cine MRI was performed to determine cardiac
function. Myocardial IR injury resulted in a similar reduction in
cardiac function at all investigated time points (Supporting
Information, Fig. S1). Ejection fractions (EF) were 52 2, 54 3
and 59 4% at day 1, week 1 and week 2, respectively, which
are lower than EF values reported for healthy mice (70–80%),
confirming the presence of myocardial infarction (22,23). Relatively
high standard deviations in EF suggested heterogeneous infarct
sizes within groups. Cardiac output (CO) and the left ventricular
mass were significantly higher at week 1 and week 2 as compared
with day 1, which can be explained by left ventricular remodeling
after IR injury.
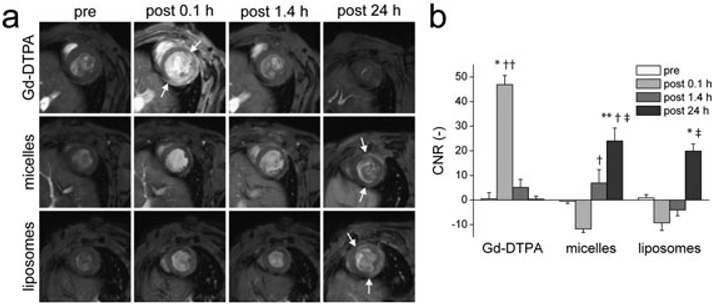
2.4. Acute IR Injury – Day 1
Gd–DTPA, micelles or liposomes were injected immediately or at
day 1 after induction of IR injury. For both groups the accumula-
tion of Gd–DTPA and paramagnetic nanoparticles was visualized
by T
1
-weighted short-axis multi-slice MRI at day 1 after the IR
injury. Injection of Gd–DTPA at day 1 resulted in immediate
hyperenhancement of the infarcted myocardium (Fig. 3a). The
hyperenhancement slowly disappeared within the next 30 min,
in agreement with the extensively described LGE effect used to
measure infarct size (24). The contrast-to-noise ratio (CNR) of
infarct versus remote tissue at 0.1 h after administration (Fig. 3b)
was significantly enhanced compared with CNRs pre injection
and at later time points (46.9 3.7, p < 0.05 vs all time points).
No residual contrast enhancement was observed at day 1 when
Gd–DTPA was injected directly after IR injury and circulated for
24 h. As a negative control for the fluorescence microscopy
analysis of hearts from nanoparticle-injected mice, CLSM of mouse
hearts injected with Gd–DTPA was performed. As expected, no
near-infrared (NIR) autofluorescence was observed (Fig. 4).
In contrast to the observations for Gd–DTPA, administration of
nanoparticles at day 1 resulted in hyperenhancement of remote
cardiac tissue at 0.1 h after injection, while the infarct area
remained isointense and, therefore, appeared as a dark rim (Fig. 3a).
Consequently, the CNR of infarct versus remote tissue at 0.1 h was
negative, amounting to 11.7 1.4 and 9.3 3.1 for micelles
and liposomes, respectively (Fig. 3b). At this early time point after
administration micelles and liposomes were still circulating in the
blood, while accumulation into infarcted myocardium might not
be present yet. We propose that the absence of signal intensity
changes in the infarct reflected the impaired perfusion of the
infarct. Often the dark rim of infarct tissue was present in the
subendocardium. Indeed, the subendocardium is most prone
to IR injury as it is located most distal from the coronary
arteries (25,26). These observations suggest that the lipid-based
Figure 2. In vivo MRI protocol. T
1
-weighted scans were acquired in all sessions to investigate accumulation of Gd–DTPA, micelles or liposomes in
myocardial tissue up to 48 h after injection. During the last imaging session of a series cine MRI scans were recorded as well, to evaluate cardiac function.
Arrows indicate the time point of injection of Gd–DTPA, micelles or liposomes. In the chronic ischemia–reperfusion (IR) injury groups, one mouse was killed
after 3 h of circulation of micelles or liposomes (day 6/13) for histology, while the other mice were followed up to 48 h after injection. IR, Ischemia–reperfusion
surgery; &, cine MRI; and †,sacrifice and tissue harvesting for histological validation.
Figure 3. In vivo MRI of acute IR injury. (a) Short-axis T
1
-weighted MR images obtained before and after injection of Gd–DTPA (row 1), micelles (row 2)
or liposomes (row 3). In columns 1–3, the agents were injected on day 1 after IR injury, while in column 4 the agents were administered at the start of
reperfusion and circulated for 24 h. Arrows indicate the areas of contrast enhancement. (b) Group-averaged CNRs between infarct and remote tissue at
different time points after injection. * p < 0.05 vs all time points; ** p < 0.05 vs pre; † p < 0.05 vs 0.1 h after injection; †† p < 0.05 vs all contrast agents at
1.4 h after injection; and { p < 0.05 vs Gd–DTPA at 24 h after injection.
LIPIDASED NANOPARTICLES IN MYOCARDIAL IR INJURY
Contrast Media Mol. Imaging 2013, 8 117–126 Copyright © 2012 John Wiley & Sons, Ltd. wileyonlinelibrary.com/journal/cmmi
119

nanoparticles may offer a surrogate diagnostic readout, as a
blood-pool agent, of the viable and perfused myocardium.
Nevertheless, for the determination of myocardial infarct size
traditional LGE MRI with Gd–DTPA is still the method of choice.
After 1.4 h, micelle accumulation in the infarct area became
apparent resulting in disappearance of the dark rim in the infarct
area (Fig. 3). The CNR increased significantly to a positive value
of 6.9 5.4 (p < 0.05 vs 0.1 h). For the larger liposomes, the
T
1
-weighted signal increase proceeded more slowly. The suben-
docardial infarct remained hypoenhanced at this time point and
this resulted in a persistent negative CNR of 4.0 2.4. CLSM
was used to detect the NIR lipid present in the micelles and
liposomes to determine nanoparticle localization at the cellular
level. For this purpose, short-axis histological sections of multiple
mice at multiple longitudinal positions and multiple locations
in the infarct, in the border zone and in the remote myocardium
were studied. CLSM of hearts at 3 h after injection confirmed
extensive accumulation of micelles in the infarcted myocar-
dium (Fig. 4, Table 2). Accumulation was high in the infarct
border zones and in the core of the infarcted myocardium. NIR
signal of micelles did not co-localize with inflammatory cells or
vessels. Staining of laminin (extracellular matrix) revealed that
micelles were primarily associated with infarcted cardiomyocytes.
Liposomes, on the other hand, were present in distinct spots in
the infarct border zone area and occasionally co-localized with
macrophages, indicating phagocytosis.
After 24 h of circulation (Fig. 3), both micelles and liposomes
had accumulated in the infarcted myocardium and caused
Figure 4. Confocal laser scanning microscopy (CLSM) of infarcted myocardium and border zones at day 1 after IR injury. Images were acquired after short
(3 h) or after long (24 h) circulation of the nanoparticles. In all images the fluorescence of NIR lipids incorporated in micelles and liposomes is shown in red. As
a fluorescence-negative control, mice injected with Gd–DTPA are shown. Scale bar = 100 mm. Row 1: green corresponds to autofluorescence of the heart.
Autofluorescence is less intense in the infarcted myocardium. Laser intensities for NIR visualization are given as percentages of the maximal laser intensity.
For NIR visualization after staining, this laser intensity was always set to 50%. Row 2: leukocytes (CD18
+
) in blue; row 3: macrophages (CD68
+
)ingreen;row4:
vessels (CD31
+
)inblue;androw5:lamininingreen.
Table 2. Visual scoring of confocal laser scanning microscopy
images for intensity of near-infrared fluorescence signal
intensities after acute ischemia–reperfusion injury
Time after
administration nanoparticle Remote Border zone Infarct
3h Gd–DTPA
Micelles +++
Liposomes +
24 h Gd–DTPA
Micelles ++
Liposomes +++
= No; = low; = moderate; + = high; and ++ = very high
near-infrared fluorescence signal intensity.
T. GEELEN ET AL.
wileyonlinelibrary.com/journal/cmmi Copyright © 2012 John Wiley & Sons, Ltd. Contrast Media Mol. Imaging 2013, 8 117–126
120