original article
The
new engl a nd jour nal
o f
medicine
n engl j med 366;22 nejm.org may 31, 2012
2074
Preoperative Chemoradiotherapy
for Esophageal or Junctional Cancer
P. van Hagen, M.C.C.M. Hulshof, J.J.B. van Lanschot, E.W. Steyerberg,
M.I. van Berge Henegouwen, B.P.L. Wijnhoven, D.J. Richel,
G.A.P. Nieuwenhuijzen, G.A.P. Hospers, J.J. Bonenkamp, M.A. Cuesta,
R.J.B. Blaisse, O.R.C. Busch, F.J.W. ten Kate, G.-J. Creemers, C.J.A. Punt,
J.T.M. Plukker, H.M.W. Verheul, E.J. Spillenaar Bilgen, H. van Dekken,
M.J.C. van der Sangen, T. Rozema, K. Biermann, J.C. Beukema,
A.H.M. Piet, C.M. van Rij, J.G. Reinders, H.W. Tilanus,
and A. van der Gaast, for the CROSS Group*
The authors’ full names, degrees, and affili-
ations are listed in the Appendix. Address
reprint requests to Dr. van der Gaast at
the Department of Medical Oncology,
Erasmus University Medical Center/Daniel
den Hoed Cancer Center, P.O. Box 2040,
3000 CA Rotterdam, the Netherlands, or at
a.vandergaast@erasmusmc.nl.
* The members of the Chemoradiothera-
py for Oesophageal Cancer Followed by
Surgery Study (CROSS) Group are list-
ed in the Supplementary Appendix,
available at NEJM.org.
N Engl J Med 2012;366:2074-84.
Copyright © 2012 Massachusetts Medical Society.
ABSTR ACT
BACKGROUND
The role of neoadjuvant chemoradiotherapy in the treatment of patients with esopha-
geal or esophagogastric-junction cancer is not well established. We compared chemo-
radiotherapy followed by surgery with surgery alone in this patient population.
METHODS
We randomly assigned patients with resectable tumors to receive surgery alone or
weekly administration of carboplatin (doses titrated to achieve an area under the
curve of 2 mg per milliliter per minute) and paclitaxel (50 mg per square meter of
body-surface area) for 5 weeks and concurrent radiotherapy (41.4 Gy in 23 fractions,
5 days per week), followed by surgery.
RESULTS
From March 2004 through December 2008, we enrolled 368 patients, 366 of whom
were included in the analysis: 275 (75%) had adenocarcinoma, 84 (23%) had squa-
mous-cell carcinoma, and 7 (2%) had large-cell undifferentiated carcinoma. Of the
366 patients, 178 were randomly assigned to chemoradiotherapy followed by surgery,
and 188 to surgery alone. The most common major hematologic toxic effects in the
chemoradiotherapy–surgery group were leukopenia (6%) and neutropenia (2%); the
most common major nonhematologic toxic effects were anorexia (5%) and fatigue (3%).
Complete resection with no tumor within 1 mm of the resection margins (R0) was
achieved in 92% of patients in the chemoradiotherapy–surgery group versus 69% in
the surgery group (P<0.001). A pathological complete response was achieved in 47 of
161 patients (29%) who underwent resection after chemoradiotherapy. Postoperative
complications were similar in the two treatment groups, and in-hospital mortality was
4% in both. Median overall survival was 49.4 months in the chemoradiotherapy–
surgery group versus 24.0 months in the surgery group. Overall survival was sig-
nificantly better in the chemoradiotherapy–surgery group (hazard ratio, 0.657; 95%
confidence interval, 0.495 to 0.871; P = 0.003).
CONCLUSIONS
Preoperative chemoradiotherapy improved survival among patients with potentially
curable esophageal or esophagogastric-junction cancer. The regimen was associated
with acceptable adverse-event rates. (Funded by the Dutch Cancer Foundation [KWF
Kankerbestrijding]; Netherlands Trial Register number, NTR487.)
The New England Journal of Medicine
Downloaded from nejm.org at VRIJE UNIVERSITEIT on October 29, 2013. For personal use only. No other uses without permission.
Copyright © 2012 Massachusetts Medical Society. All rights reserved.

Preoperative Chemoradiotherapy for Esophageal Cancer
n engl j med 366;22 nejm.org may 31, 2012
2075
W
ith new diagnoses in more than
480,000 patients annually, esophageal
cancer is the eighth most common can-
cer worldwide.
1
It is a highly lethal disease, causing
more than 400,000 deaths per year.
2
The incidence
of esophageal adenocarcinoma is rapidly rising,
whereas that of squamous-cell carcinoma remains
unchanged.
3
Despite adequate preoperative stag-
ing, 25% of patients treated with primary sur-
gery have microscopically positive resection mar-
gins (R1), and the 5-year survival rate rarely
exceeds 40%.
4
The role of neoadjuvant chemoradiotherapy has
been debated for several decades. In most random-
ized trials, no survival benefit could be shown, and
the trials were criticized for inadequate trial de-
sign, samples that were too small, and poor out-
comes in the surgery-alone group. Meta-analyses
suggest a survival benefit from neoadjuvant chemo-
radiotherapy, albeit frequently at the cost of in-
creased postoperative morbidity and mortality.
5,6
We previously reported a phase 2 trial of neo-
adjuvant chemoradiotherapy consisting of weekly
administration of carboplatin and paclitaxel with
concurrent radiotherapy.
7
This regimen was as-
sociated with a low rate of serious toxic effects,
and a complete resection with no tumor within
1 mm of the resection margins (R0) was achieved
in all patients who underwent resection. These re-
sults encouraged us to initiate a multicenter, ran-
domized, controlled, phase 3 study comparing
neoadjuvant chemoradiotherapy followed by sur-
gery with surgery alone in patients with poten-
tially curable esophageal or esophagogastric-
junction carcinoma.
8
METHODS
ELIGIBILITY CRITERIA
Patients with histologically confirmed, potentially
curable squamous-cell carcinoma, adenocarci-
noma, or large-cell undifferentiated carcinoma of
the esophagus or esophagogastric junction (i.e.,
tumors involving both the cardia and the esopha-
gus on endoscopy) were eligible for inclusion in
the study. The upper border of the tumor had to be
at least 3 cm below the upper esophageal sphincter.
Patients who had proximal gastric tumors with
minimal invasion of the esophagus were excluded.
The length and width of the tumor could not ex-
ceed 8 cm and 5 cm, respectively. Only patients
with tumors of clinical stage T1N1 or T2-3N0-1
and no clinical evidence of metastatic spread
(M0), according to the International Union
against Cancer (UICC) tumor–node–metastasis
(TNM) classification,
9
were enrolled. Eligible pa-
tients were 18 to 75 years of age, had a World
Health Organization (WHO) performance status
score of 2 or lower (on a scale of 0 to 5, with 0
indicating fully active, 1 unable to carry out
heavy physical work, and 2 up and about more
than half the day but unable to work), and had
lost 10% or less of body weight. Patients also had
to have adequate hematologic, renal, hepatic, and
pulmonary function, as well as no history of other
cancer or previous radiotherapy or chemotherapy.
All patients provided written informed consent.
The institutional review board at each participating
center approved the study protocol.
8
The protocol,
including the statistical analysis plan, is available
with the full text of this article at NEJM.org. No
commercial support was involved in the study;
the drugs were purchased. No one who is not an
author contributed to the manuscript. The first,
fourth, and last authors vouch for the accuracy
and completeness of the reported data and the
fidelity of the study to the protocol.
STAGING
All patients underwent pretreatment staging. This
included a history taking; physical examination;
pulmonary-function tests, routine hematologic
and biochemical tests; upper gastrointestinal en-
doscopy with histologic biopsy and endoscopic
ultrasonography; computed tomography of the
neck, chest, and upper abdomen; and external ul-
trasonography of the neck, with fine-needle aspi-
ration of lymph nodes when cancer was suspected.
For the final analysis, the available endoscopic re-
ports were centrally reviewed.
TREATMENT
Chemotherapy
On days 1, 8, 15, 22, and 29, carboplatin targeted
at an area under the curve of 2 mg per milliliter
per minute and paclitaxel at a dose of 50 mg per
square meter of body-surface area were adminis-
tered intravenously. All patients were intravenously
premedicated with dexamethasone, clemastine,
and ranitidine as well as standard antiemetic
agents. The patients were closely monitored for
toxic effects of chemotherapy with the use of the
National Cancer Institute’s Common Terminolo-
gy Criteria for Adverse Events, version 3.0.
10
The New England Journal of Medicine
Downloaded from nejm.org at VRIJE UNIVERSITEIT on October 29, 2013. For personal use only. No other uses without permission.
Copyright © 2012 Massachusetts Medical Society. All rights reserved.

The
ne w engl and jour nal
o f
medicine
n engl j med 366;22 nejm.org may 31, 2012
2076
Radiotherapy
A total radiation dose of 41.4 Gy was given in 23
fractions of 1.8 Gy each, with 5 fractions admin-
istered per week, starting on the first day of the
first chemotherapy cycle. All patients were treated
by means of external-beam radiation. A detailed
description of the methods of administration of
chemotherapy and radiotherapy can be found in
Appendix 1 in the Supplementary Appendix, avail-
able at NEJM.org.
Surgery
Patients in the chemoradiotherapy–surgery group
underwent surgery as soon as possible after com-
pletion of chemoradiotherapy (preferably, within
4 to 6 weeks), and patients in the surgery group
were treated as soon as possible after randomiza-
tion. A transthoracic approach with two-field
lymph-node dissection was performed for tumors
extending proximally to the tracheal bifurcation.
For tumors involving the esophagogastric junction,
a transhiatal resection was preferred. Peritruncal
dissection was carried out with both approaches.
For all other tumors, the approach depended on the
characteristics of the patient and on local prefer-
ences. Gastric-tube reconstruction with a cervical
anastomosis was the preferred technique for re-
storing the continuity of the digestive tract.
PATHOLOGICAL ANALYSIS
Reports on pathological examination had to de-
scribe the tumor type and extension, lymph nodes,
and resection margins. In the absence of macro-
scopic tumor, any abnormal-appearing tissue
was paraffin-embedded in total in order to make
an adequate assessment for the presence of re-
sidual tumor and the effects of therapy.
To grade the response to therapy, we classified
the degree of histomorphologic regression into
four categories as follows: grade 1, no evidence of
vital residual tumor cells (pathological complete
response); grade 2, less than 10% vital residual
tumor cells; grade 3, 10 to 50%; and grade 4,
more than 50%.
11,12
If a vital tumor was present
at 1 mm or less from the proximal, distal, or cir-
cumferential resection margin, it was considered
to be microscopically positive (R1).
FOLLOW-UP
During the first year after treatment was com-
pleted, patients were seen every 3 months. In the
second year, follow-up took place every 6 months,
and then at the end of each year until 5 years af-
ter treatment. Late toxic effects, disease recur-
rence, and death were documented. Recurrences
were scored at the moment of the first recur-
rence. During follow-up, diagnostic investiga-
tions were performed only when recurrence was
suspected.
STATISTICAL ANALYSIS
We calculated that 175 patients were needed in
each group in order to detect a difference in me-
dian overall survival of 22 months in the chemo-
radiotherapy–surgery group versus 16 months in
the surgery group (two-sided test; alpha level,
0.05; beta level, 0.80). Stratification factors in-
cluded histologic tumor type, treatment center,
lymph-node (N) stage as determined by endo-
scopic ultrasonography, and WHO performance
score. Block randomization was performed cen-
trally by telephone or at the central trial office,
368 Underwent randomization
837 Patients were assessed for esophageal
or EGJ cancer
469 Were excluded
2 Withdrew consent
7 Did not receive any
chemoradiotherapy
180 Were assigned to chemo-
radiotherapy and surgery
188 Were assigned to surgery
alone
178 Were included
in the analysis
188 Were included
in the analysis
171 Received chemoradiotherapy
168 Underwent surgery
161 Underwent resection
186 Underwent surgery
161 Underwent resection
Figure 1. Study Enrollment.
Of the 368 patients who underwent randomization, 178 in the chemoradio-
therapy–surgery group and 188 in the surgery group were included in the
intention-to-treat analysis. A resection was not possible in 7 patients in the
chemoradiotherapy–surgery group and in 25 in the surgery group because
the primary tumor or lymph nodes were identified as unresectable during
surgery. EGJ denotes esophagogastric junction.
The New England Journal of Medicine
Downloaded from nejm.org at VRIJE UNIVERSITEIT on October 29, 2013. For personal use only. No other uses without permission.
Copyright © 2012 Massachusetts Medical Society. All rights reserved.

Preoperative Chemoradiotherapy for Esophageal Cancer
n engl j med 366;22 nejm.org may 31, 2012
2077
according to computer-generated randomization
lists for each stratum, with random block sizes
of 4 or 6.
Data were analyzed according to the intention-
to-treat principle. The primary end point was
overall survival. All other described outcomes
were secondary end points. No post hoc analyses
were performed. Survival was calculated from
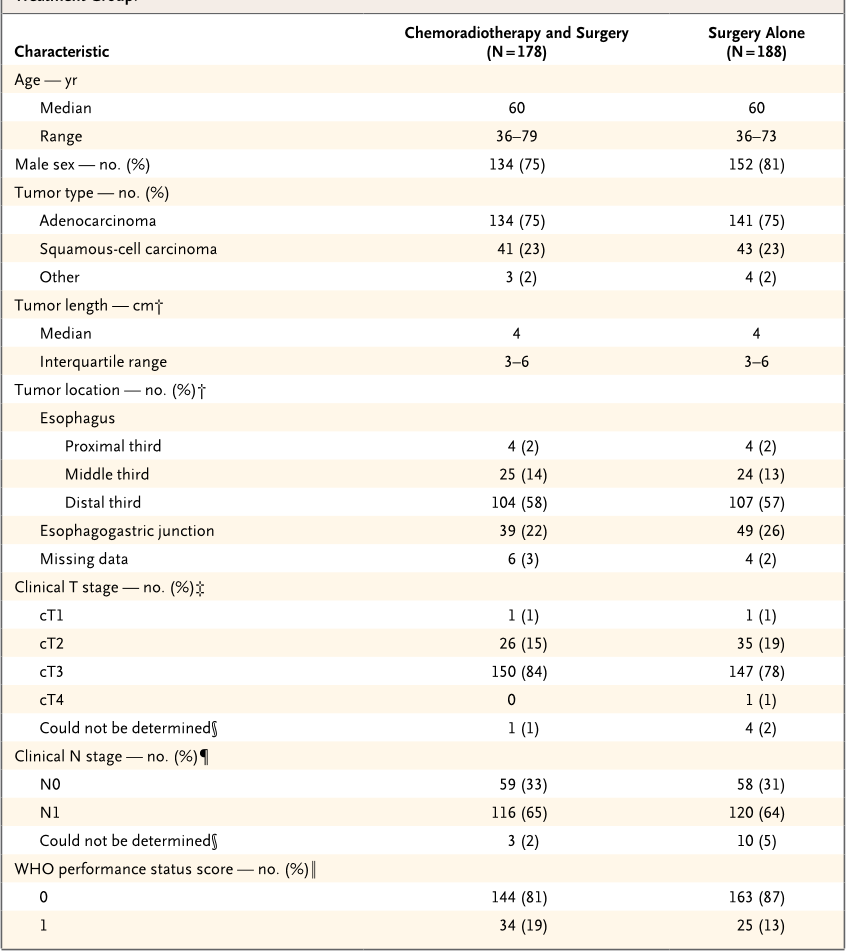
Table 1. Characteristics of Patients with Resectable Esophageal or Esophagogastric-Junction Cancer, According to
Treatment Group.*
Characteristic
Chemoradiotherapy and Surgery
(N = 178)
Surgery Alone
(N = 188)
Age — yr
Median 60 60
Range 36–79 36–73
Male sex — no. (%) 134 (75) 152 (81)
Tumor type — no. (%)
Adenocarcinoma 134 (75) 141 (75)
Squamous-cell carcinoma 41 (23) 43 (23)
Other 3 (2) 4 (2)
Tumor length — cm†
Median 4 4
Interquartile range 3–6 3–6
Tumor location — no. (%)†
Esophagus
Proximal third 4 (2) 4 (2)
Middle third 25 (14) 24 (13)
Distal third 104 (58) 107 (57)
Esophagogastric junction 39 (22) 49 (26)
Missing data 6 (3) 4 (2)
Clinical T stage — no. (%)‡
cT1 1 (1) 1 (1)
cT2 26 (15) 35 (19)
cT3 150 (84) 147 (78)
cT4 0 1 (1)
Could not be determined§ 1 (1) 4 (2)
Clinical N stage — no. (%)¶
N0 59 (33) 58 (31)
N1 116 (65) 120 (64)
Could not be determined§ 3 (2) 10 (5)
WHO performance status score — no. (%)‖
0 144 (81) 163 (87)
1 34 (19) 25 (13)
* Percentages may not add up to 100 because of rounding. WHO denotes World Health Organization.
† Tumor length and location were determined by means of endoscopy.
‡ Clinical tumor (cT) stage was assessed by means of endoscopic ultrasonography or computed tomography (CT) and was
classified according to the International Union against Cancer (UICC) tumor–node–metastasis (TNM) classification.
9
§ This category included patients in whom the tumor could not be fully investigated by means of a transducer for endoscopic
ultrasonography owing to a stenosis caused by the tumor.
¶ Clinical lymph-node (N) stage was assessed by means of endoscopic ultrasonography, CT, or
18
F-fluorodeoxyglucose
positron-emission tomography and was classified according to UICC TNM classification.
9
‖ WHO performance status scores are on a scale of 0 to 5, with lower numbers indicating better performance status; 0 indi-
cates fully active, and 1 unable to carry out heavy physical work.
The New England Journal of Medicine
Downloaded from nejm.org at VRIJE UNIVERSITEIT on October 29, 2013. For personal use only. No other uses without permission.
Copyright © 2012 Massachusetts Medical Society. All rights reserved.

The
ne w engl and jour nal
o f
medicine
n engl j med 366;22 nejm.org may 31, 2012
2078
the date of randomization until death. All data
collected through December 2010 were included
in the analysis, which guaranteed a potential
minimal follow-up of 2 years.
The Kaplan–Meier method was used to esti-
mate survival, with the log-rank test to deter-
mine significance. A Cox proportional-hazards
model was used to estimate the treatment effect
with adjustment for prognostic factors for sur-
vival. Moreover, Cox models were used to iden-
tify possible interactions in treatment effect
between subgroups, both with and without
adjustment for prognostic factors. Subgroups
were predefined according to sex, histologic
subtype of tumor, clinical N stage, and WHO
performance score. Statistical analysis was per-
formed with the use of SPSS software, version
17.0 (SPSS).
RESULTS
CHARACTERISTICS OF THE PATIENTS
From March 2004 through December 2008, we
enrolled 368 patients in the study, of whom 180
were randomly assigned to the chemoradiother-
apy–surgery group, and 188 to the surgery group.
Two patients who were randomly assigned to
the chemoradiotherapy–surgery group withdrew
consent and were not included in the analysis
(Fig. 1).
Prognostic factors were well balanced be-
tween the two treatment groups (
Table 1
). In
both groups, the median age was 60 years; 134
of 178 patients (75%) in the chemoradiotherapy–
surgery group were men, as compared with 152
of 188 patients (81%) in the surgery group. Most
patients (275 of 366 [75%]) had an adenocarci-
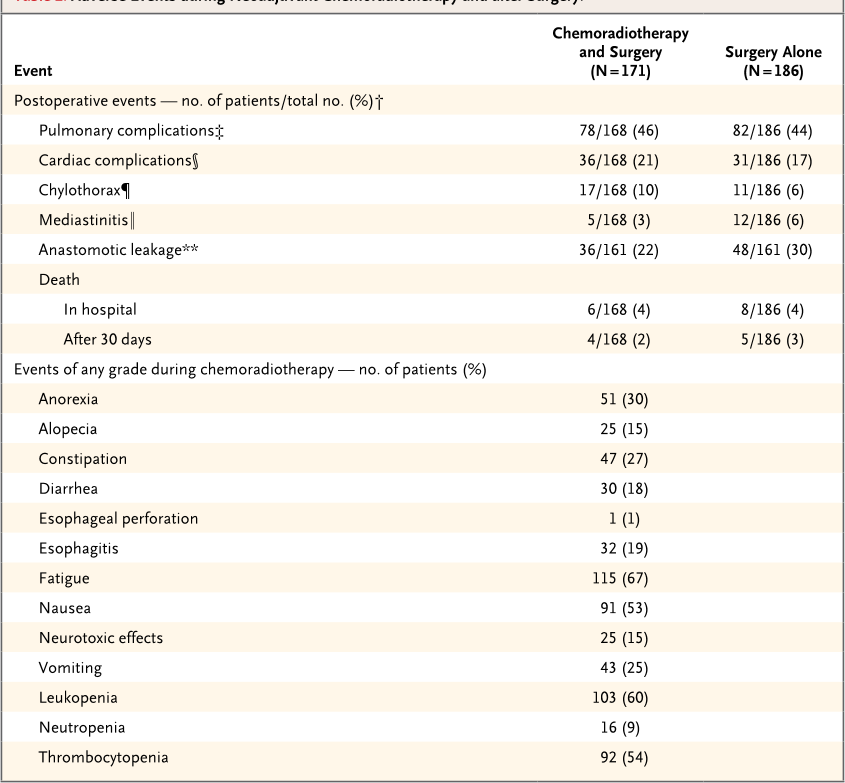
Table 2. Adverse Events during Neoadjuvant Chemoradiotherapy and after Surgery.*
Event
Chemoradiotherapy
and Surgery
(N = 171)
Surgery Alone
(N = 186)
Postoperative events — no. of patients/total no. (%)†
Pulmonary complications‡ 78/168 (46) 82/186 (44)
Cardiac complications§ 36/168 (21) 31/186 (17)
Chylothorax¶ 17/168 (10) 11/186 (6)
Mediastinitis‖ 5/168 (3) 12/186 (6)
Anastomotic leakage** 36/161 (22) 48/161 (30)
Death
In hospital 6/168 (4) 8/186 (4)
After 30 days 4/168 (2) 5/186 (3)
Events of any grade during chemoradiotherapy — no. of patients (%)
Anorexia 51 (30)
Alopecia 25 (15)
Constipation 47 (27)
Diarrhea 30 (18)
Esophageal perforation 1 (1)
Esophagitis 32 (19)
Fatigue 115 (67)
Nausea 91 (53)
Neurotoxic effects 25 (15)
Vomiting 43 (25)
Leukopenia 103 (60)
Neutropenia 16 (9)
Thrombocytopenia 92 (54)
The New England Journal of Medicine
Downloaded from nejm.org at VRIJE UNIVERSITEIT on October 29, 2013. For personal use only. No other uses without permission.
Copyright © 2012 Massachusetts Medical Society. All rights reserved.